Breakthrough COVID-19 Infection During the Delta Variant Dominant Period: Individualized Care Based on Vaccination Status Is Needed
- PMID: 35971766
- PMCID: PMC9424692
- DOI: 10.3346/jkms.2022.37.e252
Breakthrough COVID-19 Infection During the Delta Variant Dominant Period: Individualized Care Based on Vaccination Status Is Needed
Abstract
Background: The clinical features of coronavirus disease 2019 (COVID-19) patients in the COVID-19 vaccination era need to be clarified because breakthrough infection after vaccination is not uncommon.
Methods: We retrospectively analyzed hospitalized COVID-19 patients during a delta variant-dominant period 6 months after the national COVID-19 vaccination rollout. The clinical characteristics and risk factors for severe progression were assessed and subclassified according to vaccination status.
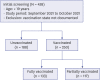
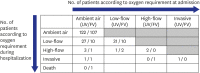
Results: A total of 438 COVID-19 patients were included; the numbers of patients in the unvaccinated, partially vaccinated and fully vaccinated groups were 188 (42.9%), 117 (26.7%) and 133 (30.4%), respectively. The vaccinated group was older, less symptomatic and had a higher Charlson comorbidity index at presentation. The proportions of patients who experienced severe progression in the unvaccinated and fully vaccinated groups were 20.3% (31/153) and 10.8% (13/120), respectively. Older age, diabetes mellitus, solid cancer, elevated levels of lactate dehydrogenase and chest X-ray abnormalities were associated with severe progression, and the vaccination at least once was the only protective factor for severe progression. Chest X-ray abnormalities at presentation were the only predictor for severe progression among fully vaccinated patients.
Conclusion: In the hospitalized setting, vaccinated and unvaccinated COVID-19 patients showed different clinical features and risk of oxygen demand despite a relatively high proportion of patients in the two groups. Vaccination needs to be assessed as an initial checkpoint, and chest X-ray may be helpful for predicting severe progression in vaccinated patients.
Keywords: Breakthrough Infection; COVID-19; Oxygen Therapy; SARS-CoV-2; Severe Progression.
© 2022 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

