The immune response as a double-edged sword: The lesson learnt during the COVID-19 pandemic
- PMID: 35971810
- PMCID: PMC9538066
- DOI: 10.1111/imm.13564
The immune response as a double-edged sword: The lesson learnt during the COVID-19 pandemic
Abstract
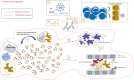
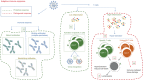
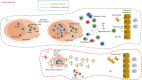
The COVID-19 pandemic has represented an unprecedented challenge for the humanity, and scientists around the world provided a huge effort to elucidate critical aspects in the fight against the pathogen, useful in designing public health strategies, vaccines and therapeutic approaches. One of the first pieces of evidence characterizing the SARS-CoV-2 infection has been its breadth of clinical presentation, ranging from asymptomatic to severe/deadly disease, and the indication of the key role played by the immune response in influencing disease severity. This review is aimed at summarizing what the SARS-CoV-2 infection taught us about the immune response, highlighting its features of a double-edged sword mediating both protective and pathogenic processes. We will discuss the protective role of soluble and cellular innate immunity and the detrimental power of a hyper-inflammation-shaped immune response, resulting in tissue injury and immunothrombotic events. We will review the importance of B- and T-cell immunity in reducing the clinical severity and their ability to cross-recognize viral variants.
Keywords: COVID-19; cross-immunity; cytokine storm; immunopathogenesis; protective immunity.
© 2022 The Authors. Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
References
-
- Long QX, Tang XJ, Shi KL, Li Q, Deng HJ, Jea Y. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26(8):1200–4. - PubMed
-
- Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–83. - PubMed
-
- Brunetta E, Folci M, Bottazzi B, de Santis M, Gritti G, Protti A, et al. Macrophage expression and prognostic significance of the long pentraxin PTX3 in COVID‐19. Nat Immunol. 2021;22:1–24. - PubMed
-
- Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2022;21:547–78. - PubMed
-
- Stravalaci M, Pagani I, Paraboschi EM, Pedotti M, Doni A, Scavello F, et al. Recognition and inhibition of SARS‐CoV‐2 by humoral innate immunity pattern recognition molecules. Nat Immunol. 2022;23:275–86. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

