The Membrane Activity of the Amphibian Temporin B Peptide Analog TB_KKG6K Sheds Light on the Mechanism That Kills Candida albicans
- PMID: 35972132
- PMCID: PMC9599520
- DOI: 10.1128/msphere.00290-22
The Membrane Activity of the Amphibian Temporin B Peptide Analog TB_KKG6K Sheds Light on the Mechanism That Kills Candida albicans
Abstract
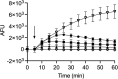
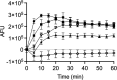
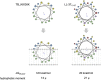
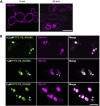
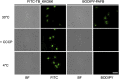
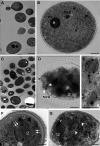
Temporin B (TB) is a 13-amino-acid-long, cationic peptide secreted by the granular glands of the European frog Rana temporaria. We recently showed that the modified TB peptide analog TB_KKG6K rapidly killed planktonic and sessile Candida albicans at low micromolar concentrations and was neither hemolytic nor cytotoxic to mammalian cells in vitro. The present study aimed to shed light into its mechanism of action, with a focus on its fungal cell membrane activity. We utilized different fluorescent dyes to prove that it rapidly induces membrane depolarization and permeabilization. Studies on model membrane systems revealed that the TB analog undergoes hydrophobic and electrostatic membrane interactions, showing a preference for anionic lipids, and identified phosphatidylinositol and cardiolipin as possible peptide targets. Fluorescence microscopy using fluorescein isothiocyanate-labeled TB_KKG6K in the presence of the lipophilic dye FM4-64 indicated that the peptide compromises membrane integrity and rapidly enters C. albicans cells in an energy-independent manner. Peptide-treated cells analyzed by cryo-based electron microscopy exhibited no signs of cell lysis; however, subcellular structures had disintegrated, suggesting that intracellular activity may form part of the killing mechanism of the peptide. Taken together, this study proved that TB_KKG6K compromises C. albicans membrane function, which explains the previously observed rapid, fungicidal mode of action and supports its great potential as a future anti-Candida therapeutic. IMPORTANCE Fungal infections with the opportunistic human pathogen C. albicans are associated with high mortality rates in immunocompromised patients. This is partly due to the yeast's ability to rapidly develop resistance toward currently available antifungals. Small, cationic, membrane-active peptides are promising compounds to fight against resistance development, as many of them effectuate rapid fungal cell death. This fast killing is believed to hamper the development of resistance, as the fungi do not have sufficient time to adapt to the antifungal compound. We previously reported that the synthetic variant of the amphibian TB peptide, TB_KKG6K, rapidly kills C. albicans. In the current study, the mechanism of action of the TB analog was investigated. We show that this TB analog is membrane-active and impairs cell membrane function, highlighting its potential to be developed as an attractive alternative anti-C. albicans therapeutic that may hinder the development of resistance.
Keywords: Candida albicans; TB analog; Temporin B; antifungal peptide; depolarization; leakage; membrane activity; permeabilization; uptake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
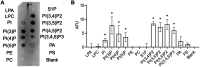
Similar articles
-
The antibacterial activity and therapeutic potential of the amphibian-derived peptide TB_KKG6K.mSphere. 2025 Jun 25;10(6):e0101624. doi: 10.1128/msphere.01016-24. Epub 2025 May 19. mSphere. 2025. PMID: 40387366 Free PMC article.
-
New Perspectives in the Antimicrobial Activity of the Amphibian Temporin B: Peptide Analogs Are Effective Inhibitors of Candida albicans Growth.J Fungi (Basel). 2021 Jun 7;7(6):457. doi: 10.3390/jof7060457. J Fungi (Basel). 2021. PMID: 34200504 Free PMC article.
-
Fungicidal mechanisms of the antimicrobial peptide Bac8c.Biochim Biophys Acta. 2015 Feb;1848(2):673-9. doi: 10.1016/j.bbamem.2014.11.024. Epub 2014 Nov 28. Biochim Biophys Acta. 2015. PMID: 25434926
-
Antifungal activity of cathelicidin peptides against planktonic and biofilm cultures of Candida species isolated from vaginal infections.Peptides. 2015 Sep;71:211-21. doi: 10.1016/j.peptides.2015.07.023. Epub 2015 Jul 31. Peptides. 2015. PMID: 26238597
-
Antifungal activity of [K3]temporin-SHa against medically relevant yeasts and moulds.Can J Microbiol. 2022 Jun;68(6):427-434. doi: 10.1139/cjm-2021-0250. Epub 2022 Mar 14. Can J Microbiol. 2022. PMID: 35286812
Cited by
-
In vitro long-term exposure to chlorhexidine or triclosan induces cross-resistance against azoles in Nakaseomyces glabratus.Antimicrob Resist Infect Control. 2025 Jan 23;14(1):2. doi: 10.1186/s13756-024-01511-4. Antimicrob Resist Infect Control. 2025. PMID: 39849551 Free PMC article.
-
Bactericidal Activity to Escherichia coli: Different Modes of Action of Two 24-Mer Peptides SAAP-148 and OP-145, Both Derived from Human Cathelicidine LL-37.Antibiotics (Basel). 2023 Jul 8;12(7):1163. doi: 10.3390/antibiotics12071163. Antibiotics (Basel). 2023. PMID: 37508259 Free PMC article.
-
Octenidine effectively reduces Candida auris colonisation on human skin.Sci Rep. 2025 Jul 25;15(1):27034. doi: 10.1038/s41598-025-11914-x. Sci Rep. 2025. PMID: 40715367 Free PMC article.
-
Temporins: Multifunctional Peptides from Frog Skin.Int J Mol Sci. 2023 Mar 12;24(6):5426. doi: 10.3390/ijms24065426. Int J Mol Sci. 2023. PMID: 36982501 Free PMC article. Review.
-
Octenidine's Efficacy: A Matter of Interpretation or the Influence of Experimental Setups?Antibiotics (Basel). 2022 Nov 19;11(11):1665. doi: 10.3390/antibiotics11111665. Antibiotics (Basel). 2022. PMID: 36421309 Free PMC article.
References
-
- do Nascimento Dias J, de Souza Silva C, de Araújo AR, Souza JMT, de Holanda Veloso Júnior PH, Cabral WF, da Glória da Silva M, Eaton P, de Souza de Almeida Leite JR, Nicola AM, Albuquerque P, Silva-Pereira I. 2020. Mechanisms of action of antimicrobial peptides ToAP2 and NDBP-5.7 against Candida albicans planktonic and biofilm cells. Sci Rep 10:10327. doi:10.1038/s41598-020-67041-2. - DOI - PMC - PubMed

