Evidence for a Widespread Third System for Bacterial Polysaccharide Export across the Outer Membrane Comprising a Composite OPX/β-Barrel Translocon
- PMID: 35972145
- PMCID: PMC9601211
- DOI: 10.1128/mbio.02032-22
Evidence for a Widespread Third System for Bacterial Polysaccharide Export across the Outer Membrane Comprising a Composite OPX/β-Barrel Translocon
Abstract
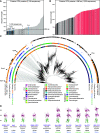
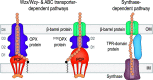
In Gram-negative bacteria, secreted polysaccharides have multiple critical functions. In Wzx/Wzy- and ABC transporter-dependent pathways, an outer membrane (OM) polysaccharide export (OPX) type translocon exports the polysaccharide across the OM. The paradigm OPX protein Wza of Escherichia coli is an octamer in which the eight C-terminal domains form an α-helical OM pore and the eight copies of the three N-terminal domains (D1 to D3) form a periplasmic cavity. In synthase-dependent pathways, the OM translocon is a 16- to 18-stranded β-barrel protein. In Myxococcus xanthus, the secreted polysaccharide EPS (exopolysaccharide) is synthesized in a Wzx/Wzy-dependent pathway. Here, using experiments, phylogenomics, and computational structural biology, we identify and characterize EpsX as an OM 18-stranded β-barrel protein important for EPS synthesis and identify AlgE, a β-barrel translocon of a synthase-dependent pathway, as its closest structural homolog. We also find that EpsY, the OPX protein of the EPS pathway, consists only of the periplasmic D1 and D2 domains and completely lacks the domain for spanning the OM (herein termed a D1D2OPX protein). In vivo, EpsX and EpsY mutually stabilize each other and interact in in vivo pulldown experiments supporting their direct interaction. Based on these observations, we propose that EpsY and EpsX make up and represent a third type of translocon for polysaccharide export across the OM. Specifically, in this composite translocon, EpsX functions as the OM-spanning β-barrel translocon together with the periplasmic D1D2OPX protein EpsY. Based on computational genomics, similar composite systems are widespread in Gram-negative bacteria. IMPORTANCE Bacteria secrete a wide variety of polysaccharides that have critical functions in, e.g., fitness, surface colonization, and biofilm formation and in beneficial and pathogenic human-, animal-, and plant-microbe interactions. In Gram-negative bacteria, export of these chemically diverse polysaccharides across the outer membrane depends on two known translocons, i.e., an outer membrane OPX protein in Wzx/Wzy- and ABC transporter-dependent pathways and an outer membrane 16- to 18-stranded β-barrel protein in synthase-dependent pathways. Here, using a combination of experiments in Myxococcus xanthus, phylogenomics, and computational structural biology, we provide evidence supporting that a third type of translocon can export polysaccharides across the outer membrane. Specifically, in this translocon, an outer membrane-spanning β-barrel protein functions together with an entirely periplasmic OPX protein that completely lacks the domain for spanning the OM. Computational genomics support that similar composite systems are widespread in Gram-negative bacteria.
Keywords: Myxococcus xanthus; OM translocon; OPX proteins; Wzx/Wzy pathway; beta-barrel proteins; capsular polysaccharide; exopolysaccharide; export of polysaccharides; synthase-dependent pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Morris G, Harding S. 2009. Polysaccharides, microbial, p 482–494. In Encyclopedia of microbiology. Elsevier. doi: 10.1016/B978-012373944-5.00135-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

