Herpes Simplex Virus 1 Entry Glycoproteins Form Complexes before and during Membrane Fusion
- PMID: 35972147
- PMCID: PMC9600979
- DOI: 10.1128/mbio.02039-22
Herpes Simplex Virus 1 Entry Glycoproteins Form Complexes before and during Membrane Fusion
Abstract
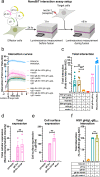
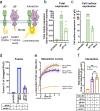
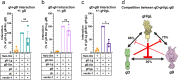
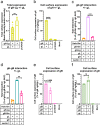
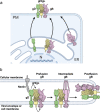
Herpesviruses-ubiquitous pathogens that cause persistent infections-have some of the most complex cell entry mechanisms. Entry of the prototypical herpes simplex virus 1 (HSV-1) requires coordinated efforts of 4 glycoproteins, gB, gD, gH, and gL. The current model posits that the glycoproteins do not interact before receptor engagement and that binding of gD to its receptor causes a "cascade" of sequential pairwise interactions, first activating the gH/gL complex and subsequently activating gB, the viral fusogen. But how these glycoproteins interact remains unresolved. Here, using a quantitative split-luciferase approach, we show that pairwise HSV-1 glycoprotein complexes form before fusion, interact at a steady level throughout fusion, and do not depend on the presence of the cellular receptor. Based on our findings, we propose a revised "conformational cascade" model of HSV-1 entry. We hypothesize that all 4 glycoproteins assemble into a complex before fusion, with gH/gL positioned between gD and gB. Once gD binds to a cognate receptor, the proximity of the glycoproteins within this complex allows for efficient transmission of the activating signal from the receptor-activated gD to gH/gL to gB through sequential conformational changes, ultimately triggering the fusogenic refolding of gB. Our results also highlight previously unappreciated contributions of the transmembrane and cytoplasmic domains to glycoprotein interactions and fusion. Similar principles could be at play in other multicomponent viral entry systems, and the split-luciferase approach used here is a powerful tool for investigating protein-protein interactions in these and a variety of other systems. IMPORTANCE Herpes simplex virus 1 (HSV-1) infects the majority of humans for life and can cause diseases ranging from painful sores to deadly brain inflammation. No vaccines or curative treatments currently exist. HSV-1 infection of target cells requires coordinated efforts of four viral glycoproteins. But how these glycoproteins interact remains unclear. Using a quantitative protein interaction assay, we found that HSV-1 glycoproteins form receptor-independent complexes and interact at a steady level. We propose that the 4 proteins form a complex, which could facilitate transmission of the entry-triggering signal from the receptor-binding component to the membrane fusogen component through sequential conformational changes. Similar principles could be applicable across other multicomponent protein systems. A revised model of HSV-1 entry could facilitate the development of therapeutics targeting this process.
Keywords: HSV-1; NanoBiT protein interaction assay; chimeric protein; domain; glycoprotein; herpesvirus; interaction; membrane fusion; split-luciferase cell-cell fusion assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Multiple Sites on Glycoprotein H (gH) Functionally Interact with the gB Fusion Protein to Promote Fusion during Herpes Simplex Virus (HSV) Entry.mBio. 2023 Feb 28;14(1):e0336822. doi: 10.1128/mbio.03368-22. Epub 2023 Jan 11. mBio. 2023. PMID: 36629412 Free PMC article.
-
Characterization of Vesicular Stomatitis Virus Pseudotypes Bearing Essential Entry Glycoproteins gB, gD, gH, and gL of Herpes Simplex Virus 1.J Virol. 2016 Oct 28;90(22):10321-10328. doi: 10.1128/JVI.01714-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27605677 Free PMC article.
-
A Functional Interaction between Herpes Simplex Virus 1 Glycoprotein gH/gL Domains I and II and gD Is Defined by Using Alphaherpesvirus gH and gL Chimeras.J Virol. 2015 Jul;89(14):7159-69. doi: 10.1128/JVI.00740-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926636 Free PMC article.
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
-
The Role of HSV Glycoproteins in Mediating Cell Entry.Adv Exp Med Biol. 2018;1045:3-21. doi: 10.1007/978-981-10-7230-7_1. Adv Exp Med Biol. 2018. PMID: 29896660 Review.
Cited by
-
A Single Amino Acid Substitution in the Transmembrane Domain of Glycoprotein H Functionally Compensates for the Absence of gL in Pseudorabies Virus.Viruses. 2023 Dec 22;16(1):26. doi: 10.3390/v16010026. Viruses. 2023. PMID: 38257727 Free PMC article.
-
Impact of actin polymerization and filopodia formation on herpes simplex virus entry in epithelial, neuronal, and T lymphocyte cells.Front Cell Infect Microbiol. 2023 Nov 24;13:1301859. doi: 10.3389/fcimb.2023.1301859. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38076455 Free PMC article. Review.
-
Viral fusion proteins of classes II and III recognize and reorganize complex biological membranes.Commun Biol. 2025 May 9;8(1):717. doi: 10.1038/s42003-025-08040-9. Commun Biol. 2025. PMID: 40341632 Free PMC article.
-
Viral Membrane Fusion: A Dance Between Proteins and Lipids.Annu Rev Virol. 2023 Sep 29;10(1):139-161. doi: 10.1146/annurev-virology-111821-093413. Annu Rev Virol. 2023. PMID: 37774128 Free PMC article. Review.
-
N-Linked Glycosylation and Expression of Duck Plague Virus pUL10 Promoted by pUL49.5.Microbiol Spectr. 2023 Aug 17;11(4):e0162523. doi: 10.1128/spectrum.01625-23. Epub 2023 Jun 28. Microbiol Spectr. 2023. PMID: 37378543 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

