Finerenone in Patients With Chronic Kidney Disease and Type 2 Diabetes by Sodium-Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis
- PMID: 35972218
- PMCID: PMC9862372
- DOI: 10.2337/dc22-0294
Finerenone in Patients With Chronic Kidney Disease and Type 2 Diabetes by Sodium-Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis
Abstract
Objective: Finerenone reduced the risk of kidney and cardiovascular events in people with chronic kidney disease (CKD) and type 2 diabetes in the FIDELIO-DKD and FIGARO-DKD phase 3 studies. Effects of finerenone on outcomes in patients taking sodium-glucose cotransporter 2 inhibitors (SGLT2is) were evaluated in a prespecified pooled analysis of these studies.
Research design and methods: Patients with type 2 diabetes and urine albumin-to-creatinine ratio (UACR) ≥30 to ≤5,000 mg/g and estimated glomerular filtration rate (eGFR) ≥25 mL/min/1.73 m2 were randomly assigned to finerenone or placebo; SGLT2is were permitted at any time. Outcomes included cardiovascular composite (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and kidney composite (kidney failure, sustained ≥57% eGFR decline, or renal death) end points, changes in UACR and eGFR, and safety outcomes.
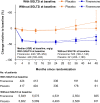
Results: Among 13,026 patients, 877 (6.7%) received an SGLT2i at baseline and 1,113 (8.5%) initiated one during the trial. For the cardiovascular composite, the hazard ratios (HRs) were 0.87 (95% CI 0.79-0.96) without SGLT2i and 0.67 (95% CI 0.42-1.07) with SGLT2i. For the kidney composite, the HRs were 0.80 (95% CI 0.69-0.92) without SGLT2i and 0.42 (95% CI 0.16-1.08) with SGLT2i. Baseline SGLT2i use did not affect risk reduction for the cardiovascular or kidney composites with finerenone (Pinteraction = 0.46 and 0.29, respectively); neither did SGLT2i use concomitant with study treatment.
Conclusions: Benefits of finerenone compared with placebo on cardiorenal outcomes in patients with CKD and type 2 diabetes were observed irrespective of SGLT2i use.
© 2022 by the American Diabetes Association.
Figures

Comment in
-
Complementary actions of finerenone and SGLT2-i on renal outcomes?: An urgent need for more information.Nephrology (Carlton). 2022 Dec;27(12):915-916. doi: 10.1111/nep.14124. Epub 2022 Oct 22. Nephrology (Carlton). 2022. PMID: 36273253 Free PMC article. No abstract available.
-
What Is the Best Medicine for Chronic Kidney Disease in Diabetes?Diabetes Care. 2022 Dec 1;45(12):2814-2816. doi: 10.2337/dci22-0029. Diabetes Care. 2022. PMID: 36455128 No abstract available.
References
-
- United States Renal Data System . Epidemiology of kidney disease in the United States. USRDS Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2020. Accessed 15 November 2021. Available from https://adr.usrds.org/2020
-
- American Diabetes Association . 10. Cardiovascular disease and risk management: standards of medical care in diabetes–2020. Diabetes Care 2020;43(Suppl. 1):S111–S134 - PubMed
-
- Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98:S1–S115 - PubMed
-
- Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

