Engineered Bacteriophages Containing Anti-CRISPR Suppress Infection of Antibiotic-Resistant P. aeruginosa
- PMID: 35972246
- PMCID: PMC9602763
- DOI: 10.1128/spectrum.01602-22
Engineered Bacteriophages Containing Anti-CRISPR Suppress Infection of Antibiotic-Resistant P. aeruginosa
Abstract
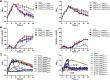
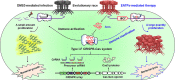
The therapeutic use of bacteriophages (phages) provides great promise for treating multidrug-resistant (MDR) bacterial infections. However, an incomplete understanding of the interactions between phages and bacteria has negatively impacted the application of phage therapy. Here, we explored engineered anti-CRISPR (Acr) gene-containing phages (EATPs, eat Pseudomonas) by introducing Type I anti-CRISPR (AcrIF1, AcrIF2, and AcrIF3) genes into the P. aeruginosa bacteriophage DMS3/DMS3m to render the potential for blocking P. aeruginosa replication and infection. In order to achieve effective antibacterial activities along with high safety against clinically isolated MDR P. aeruginosa through an anti-CRISPR immunity mechanism in vitro and in vivo, the inhibitory concentration for EATPs was 1 × 108 PFU/mL with a multiplicity of infection value of 0.2. In addition, the EATPs significantly suppressed the antibiotic resistance caused by a highly antibiotic-resistant PA14 infection. Collectively, these findings provide evidence that engineered phages may be an alternative, viable approach by which to treat patients with an intractable bacterial infection, especially an infection by clinically MDR bacteria that are unresponsive to conventional antibiotic therapy. IMPORTANCE Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic Gram-negative bacterium that causes severe infection in immune-weakened individuals, especially patients with cystic fibrosis, burn wounds, cancer, or chronic obstructive pulmonary disease (COPD). Treating P. aeruginosa infection with conventional antibiotics is difficult due to its intrinsic multidrug resistance. Engineered bacteriophage therapeutics, acting as highly viable alternative treatments of multidrug-resistant (MDR) bacterial infections, have great potential to break through the evolutionary constraints of bacteriophages to create next-generation antimicrobials. Here, we found that engineered anti-CRISPR (Acr) gene-containing phages (EATPs, eat Pseudomonas) display effective antibacterial activities along with high safety against clinically isolated MDR P. aeruginosa through an anti-CRISPR immunity mechanism in vitro and in vivo. EATPs also significantly suppressed the antibiotic resistance caused by a highly antibiotic-resistant PA14 infection, which may provide novel insight toward developing bacteriophages to treat patients with intractable bacterial infections, especially infections by clinically MDR bacteria that are unresponsive to conventional antibiotic therapy.
Keywords: Acr; CRISPR-Cas; P. aeruginosa; anti-CRISPR; bacteriophages; multidrug resistance bacterial infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Isolation and Characterization of Three Pseudomonas aeruginosa Viruses with Therapeutic Potential.Microbiol Spectr. 2023 Jun 15;11(3):e0463622. doi: 10.1128/spectrum.04636-22. Epub 2023 May 1. Microbiol Spectr. 2023. PMID: 37125933 Free PMC article.
-
Genomic variation in Pseudomonas aeruginosa clinical respiratory isolates with de novo resistance to a bacteriophage cocktail.Microbiol Spectr. 2025 May 6;13(5):e0214924. doi: 10.1128/spectrum.02149-24. Epub 2025 Mar 31. Microbiol Spectr. 2025. PMID: 40162801 Free PMC article.
-
Characterization of a new Pseudomonas aeruginosa Queuovirinae bacteriophage.Microbiol Spectr. 2024 Mar 5;12(3):e0371923. doi: 10.1128/spectrum.03719-23. Epub 2024 Feb 12. Microbiol Spectr. 2024. PMID: 38345389 Free PMC article.
-
Pseudomonas aeruginosa Bacteriophages and Their Clinical Applications.Viruses. 2024 Jun 29;16(7):1051. doi: 10.3390/v16071051. Viruses. 2024. PMID: 39066214 Free PMC article. Review.
-
Phage-Based Therapy in Combination with Antibiotics: A Promising Alternative against Multidrug-Resistant Gram-Negative Pathogens.Pathogens. 2024 Oct 14;13(10):896. doi: 10.3390/pathogens13100896. Pathogens. 2024. PMID: 39452768 Free PMC article. Review.
Cited by
-
New Antimicrobial Strategies to Treat Multi-Drug Resistant Infections Caused by Gram-Negatives in Cystic Fibrosis.Antibiotics (Basel). 2024 Jan 11;13(1):71. doi: 10.3390/antibiotics13010071. Antibiotics (Basel). 2024. PMID: 38247630 Free PMC article. Review.
-
Improving phage therapy by evasion of phage resistance mechanisms.JAC Antimicrob Resist. 2024 Feb 9;6(1):dlae017. doi: 10.1093/jacamr/dlae017. eCollection 2024 Feb. JAC Antimicrob Resist. 2024. PMID: 38343627 Free PMC article. Review.
-
Advances in CRISPR-Cas technology and its applications: revolutionising precision medicine.Front Genome Ed. 2024 Dec 12;6:1509924. doi: 10.3389/fgeed.2024.1509924. eCollection 2024. Front Genome Ed. 2024. PMID: 39726634 Free PMC article. Review.
-
Phage and Endolysin Therapy Against Antibiotics Resistant Bacteria: From Bench to Bedside.MedComm (2020). 2025 Jul 13;6(7):e70280. doi: 10.1002/mco2.70280. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40661138 Free PMC article. Review.
-
Structure-guided discovery of anti-CRISPR and anti-phage defense proteins.Nat Commun. 2024 Jan 20;15(1):649. doi: 10.1038/s41467-024-45068-7. Nat Commun. 2024. PMID: 38245560 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

