UPEC Colonic-Virulence and Urovirulence Are Blunted by Proanthocyanidins-Rich Cranberry Extract Microbial Metabolites in a Gut Model and a 3D Tissue-Engineered Urothelium
- PMID: 35972287
- PMCID: PMC9603664
- DOI: 10.1128/spectrum.02432-21
UPEC Colonic-Virulence and Urovirulence Are Blunted by Proanthocyanidins-Rich Cranberry Extract Microbial Metabolites in a Gut Model and a 3D Tissue-Engineered Urothelium
Abstract
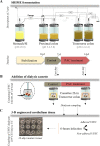
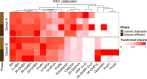
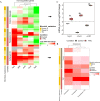
Uropathogenic Escherichia coli (UPEC) ecology-pathophysiology from the gut reservoir to its urothelium infection site is poorly understood, resulting in equivocal benefits in the use of cranberry as prophylaxis against urinary tract infections. To add further understanding from the previous findings on PAC antiadhesive properties against UPEC, we assessed in this study the effects of proanthocyanidins (PAC) rich cranberry extract microbial metabolites on UTI89 virulence and fitness in contrasting ecological UPEC's environments. For this purpose, we developed an original model combining a colonic fermentation system (SHIME) with a dialysis cassette device enclosing UPEC and a 3D tissue-engineered urothelium. Two healthy fecal donors inoculated the colons. Dialysis cassettes containing 7log10 CFU/mL UTI89 were immersed for 2h in the SHIME colons to assess the effect of untreated (7-day control diet)/treated (14-day PAC-rich extract) metabolomes on UPEC behavior. Engineered urothelium were then infected with dialysates containing UPEC for 6 h. This work demonstrated for the first time that in the control fecal microbiota condition without added PAC, the UPEC virulence genes were activated upstream the infection site, in the gut. However, PAC microbial-derived cranberry metabolites displayed a remarkable propensity to blunt activation of genes encoding toxin, adhesin/invasins in the gut and on the urothelium, in a donor-dependent manner. Variability in subjects' gut microbiota and ensuing contrasting cranberry PAC metabolism affects UPEC virulence and should be taken into consideration when designing cranberry efficacy clinical trials. IMPORTANCE Uropathogenic Escherichia coli (UPEC) are the primary cause of recurrent urinary tract infections (UTI). The poor understanding of UPEC ecology-pathophysiology from its reservoir-the gut, to its infection site-the urothelium, partly explains the inadequate and abusive use of antibiotics to treat UTI, which leads to a dramatic upsurge in antibiotic-resistance cases. In this context, we evaluated the effect of a cranberry proanthocyanidins (PAC)-rich extract on the UPEC survival and virulence in a bipartite model of a gut microbial environment and a 3D urothelium model. We demonstrated that PAC-rich cranberry extract microbial metabolites significantly blunt activation of UPEC virulence genes at an early stage in the gut reservoir. We also showed that altered virulence in the gut affects infectivity on the urothelium in a microbiota-dependent manner. Among the possible mechanisms, we surmise that specific microbial PAC metabolites may attenuate UPEC virulence, thereby explaining the preventative, yet contentious properties of cranberry against UTI.
Keywords: PAC; UPEC; cranberry; gut metabolome; urinary tract infections; urothelium; virulence genes.
Conflict of interest statement
The authors declare a conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Y.D. hold the NSERC-DianaFood Industrial Research Chair (IRC) on the prebiotic effect of polyphenols in fruits and vegetables. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures



References
-
- Nielsen KL, Stegger M, Kiil K, Godfrey PA, Feldgarden M, Lilje B, Andersen PS, Frimodt-Moller N. 2017. Whole-genome comparison of urinary pathogenic Escherichia coli and faecal isolates of UTI patients and healthy controls. Int J Med Microbiol 307:497–507. doi:10.1016/j.ijmm.2017.09.007. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

