Stitching and registering highly multiplexed whole-slide images of tissues and tumors using ASHLAR
- PMID: 35972352
- PMCID: PMC9525007
- DOI: 10.1093/bioinformatics/btac544
Stitching and registering highly multiplexed whole-slide images of tissues and tumors using ASHLAR
Abstract
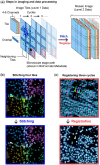
Motivation: Stitching microscope images into a mosaic is an essential step in the analysis and visualization of large biological specimens, particularly human and animal tissues. Recent approaches to highly multiplexed imaging generate high-plex data from sequential rounds of lower-plex imaging. These multiplexed imaging methods promise to yield precise molecular single-cell data and information on cellular neighborhoods and tissue architecture. However, attaining mosaic images with single-cell accuracy requires robust image stitching and image registration capabilities that are not met by existing methods.
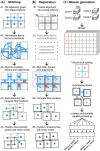
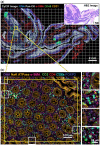
Results: We describe the development and testing of ASHLAR, a Python tool for coordinated stitching and registration of 103 or more individual multiplexed images to generate accurate whole-slide mosaics. ASHLAR reads image formats from most commercial microscopes and slide scanners, and we show that it performs better than existing open-source and commercial software. ASHLAR outputs standard OME-TIFF images that are ready for analysis by other open-source tools and recently developed image analysis pipelines.
Availability and implementation: ASHLAR is written in Python and is available under the MIT license at https://github.com/labsyspharm/ashlar. The newly published data underlying this article are available in Sage Synapse at https://dx.doi.org/10.7303/syn25826362; the availability of other previously published data re-analyzed in this article is described in Supplementary Table S4. An informational website with user guides and test data is available at https://labsyspharm.github.io/ashlar/.
Supplementary information: Supplementary data are available at Bioinformatics online.
© The Author(s) 2022. Published by Oxford University Press.
Figures



References
-
- Center for Devices and Radiological Health. (2015) Technical Performance Assessment of Digital Pathology Whole Slide Imaging Devices. US Food and Drug Administration. FDA-2015-D-0230.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

