Synthesis of Tertiary Amines through Extrusive Alkylation of Carbamates
- PMID: 35972395
- PMCID: PMC9420822
- DOI: 10.1021/acs.orglett.2c02516
Synthesis of Tertiary Amines through Extrusive Alkylation of Carbamates
Abstract
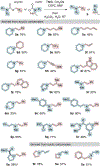
Basic amines are key elements of many biologically active natural products and pharmaceuticals. Given their inherent reactivity, it is often necessary to protect basic amines during target-directed synthesis, which results in wasteful protection/deprotection sequences. We report a step-economical approach enabling the protection of secondary amines as carbamates prior to their conversion to tertiary amines via the formal extrusion of CO2. This method is applied to the synthesis of iboga alkaloids (±)-conodusine A and (±)-conodusine B.
Figures


References
-
- Zapata F; Matey JM; Montalvo G; García-Ruiz C. Chemical Classification of New Psychoactive Substances (NPS). Microchemical Journal 2021, 163, 105877.
-
- Brotzel F; Chu YC; Mayr H. Nucleophilicities of Primary and Secondary Amines in Water. J. Org. Chem 2007, 72 (10), 3679–3688. - PubMed
-
- Du Y; Chen H; Chen R; Xu N. Poisoning effect of some nitrogen compounds on nano-sized nickel catalysts in p-nitrophenol hydrogenation. Chemical Engineering Journal, 2006, 125, 9–14.
-
- Li M-L; Yu J-H; Li Y-H; Zhu S-F; Zhou Q-L Highly Enantioselective Carbene Insertion into N–H Bonds of Aliphatic Amines. Science 2019, 366 (6468), 990–994. - PubMed
-
- Bidlingmeyer BA; Del Rios JK; Korpi J. Separation of Organic Amine Compounds on Silica Gel with Reversed-Phase Eluents. Anal. Chem 1982, 54 (3), 442–447.

