Site-dependent shaping of field potential waveforms
- PMID: 35972425
- PMCID: PMC10068269
- DOI: 10.1093/cercor/bhac297
Site-dependent shaping of field potential waveforms
Abstract
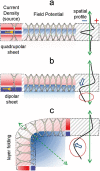
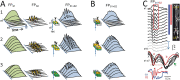
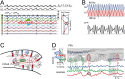
The activity of neuron populations gives rise to field potentials (FPs) that extend beyond the sources. Their mixing in the volume dilutes the original temporal motifs in a site-dependent manner, a fact that has received little attention. And yet, it potentially rids of physiological significance the time-frequency parameters of individual waves (amplitude, phase, duration). This is most likely to happen when a single source or a local origin is erroneously assumed. Recent studies using spatial treatment of these signals and anatomically realistic modeling of neuron aggregates provide convincing evidence for the multisource origin and site-dependent blend of FPs. Thus, FPs generated in primary structures like the neocortex and hippocampus reach far and cross-contaminate each other but also, they add and even impose their temporal traits on distant regions. Furthermore, both structures house neurons that act as spatially distinct (but overlapped) FP sources whose activation is state, region, and time dependent, making the composition of so-called local FPs highly volatile and strongly site dependent. Since the spatial reach cannot be predicted without source geometry, it is important to assess whether waveforms and temporal motifs arise from a single source; otherwise, those from each of the co-active sources should be sought.
Keywords: FP sources; LFPs; network oscillations; source mixing; volume conduction.
© The Author(s) 2022. Published by Oxford University Press.
Figures




References
-
- Andersen P, Bliss TVP, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971:13:222–238. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996:273:1868–1871. - PubMed
-
- Bell A, Sejnowski T. An information-maximization approach to blind separation and blind deconvolution. Neur Comp. 1995:7:1129–1159. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

