Drug metabolism and drug transport of the 100 most prescribed oral drugs
- PMID: 35972991
- PMCID: PMC9804310
- DOI: 10.1111/bcpt.13780
Drug metabolism and drug transport of the 100 most prescribed oral drugs
Abstract
Safe and effective use of drugs requires an understanding of metabolism and transport. We identified the 100 most prescribed drugs in six countries and conducted a literature search on in vitro data to assess contribution of Phase I and II enzymes and drug transporters to metabolism and transport. Eighty-nine of the 100 drugs undergo drug metabolism or are known substrates for drug transporters. Phase I enzymes are involved in metabolism of 67 drugs, while Phase II enzymes mediate metabolism of 18 drugs. CYP3A4/5 is the most important Phase I enzyme involved in metabolism of 43 drugs followed by CYP2D6 (23 drugs), CYP2C9 (23 drugs), CYP2C19 (22 drugs), CYP1A2 (14 drugs) and CYP2C8 (11 drugs). More than half of the drugs (54 drugs) are known substrates for drug transporters. P-glycoprotein (P-gp) is known to be involved in transport of 30 drugs, while breast cancer resistance protein (BCRP) facilitates transport of 11 drugs. A considerable proportion of drugs are subject to a combination of Phase I metabolism, Phase II metabolism and/or drug transport. We conclude that the majority of the most frequently prescribed drugs depend on drug metabolism or drug transport. Thus, understanding variability of drug metabolism and transport remains a priority.
Keywords: ADME; CYP3A4; P-gp; drug metabolism; drug transport.
© 2022 The Authors. Basic & Clinical Pharmacology & Toxicology published by John Wiley & Sons Ltd on behalf of Nordic Association for the Publication of BCPT (former Nordic Pharmacological Society).
Conflict of interest statement
Ann‐Cathrine Dalgård Dunvald has given paid lectures for Astellas Pharma. Tore B. Stage has given paid lectures for Pfizer and Eisai and done consulting for Pfizer. Anton Pottegård has participated in research projects funded by Alcon, Almirall, Astellas, AstraZeneca, Boehringer‐Ingelheim, Novo Nordisk, Servier and LEO Pharma, all regulator‐mandated Phase IV studies. All of this is unrelated to the work done in this review. Ditte Bork Iversen and Nanna Elman Andersen declare that they have no conflict of interest.
Figures
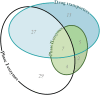
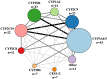

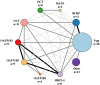

References
-
- Kornholt J, Christensen MB. Prevalence of polypharmacy in Denmark. Dan Med J. 2020;67(6):A12190680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

