Early Stages of RNA-Mediated Conversion of Human Prions
- PMID: 35973105
- PMCID: PMC9420815
- DOI: 10.1021/acs.jpcb.2c04614
Early Stages of RNA-Mediated Conversion of Human Prions
Abstract
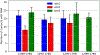
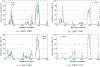
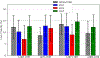
Prion diseases are characterized by the conversion of prion proteins from a PrPC fold into a disease-causing PrPSC form that is self-replicating. A possible agent to trigger this conversion is polyadenosine RNA, but both mechanism and pathways of the conversion are poorly understood. Using coarse-grained molecular dynamic simulations we study the time evolution of PrPC over 600 μs. We find that both the D178N mutation and interacting with polyadenosine RNA reduce the helicity of the protein and encourage formation of segments with strand-like motifs. We conjecture that these transient β-strands nucleate the conversion of the protein to the scrapie conformation PrPSC.
Figures


Similar articles
-
Virus Infection, Genetic Mutations, and Prion Infection in Prion Protein Conversion.Int J Mol Sci. 2021 Nov 18;22(22):12439. doi: 10.3390/ijms222212439. Int J Mol Sci. 2021. PMID: 34830321 Free PMC article. Review.
-
Recombinant human prion protein inhibits prion propagation in vitro.Sci Rep. 2013 Oct 9;3:2911. doi: 10.1038/srep02911. Sci Rep. 2013. PMID: 24105336 Free PMC article.
-
Strain-Dependent Prion Infection in Mice Expressing Prion Protein with Deletion of Central Residues 91-106.Int J Mol Sci. 2020 Oct 1;21(19):7260. doi: 10.3390/ijms21197260. Int J Mol Sci. 2020. PMID: 33019549 Free PMC article.
-
Co-existence of distinct prion types enables conformational evolution of human PrPSc by competitive selection.J Biol Chem. 2013 Oct 11;288(41):29846-61. doi: 10.1074/jbc.M113.500108. Epub 2013 Aug 23. J Biol Chem. 2013. PMID: 23974118 Free PMC article.
-
Transition of the prion protein from a structured cellular form (PrPC ) to the infectious scrapie agent (PrPSc ).Protein Sci. 2019 Dec;28(12):2055-2063. doi: 10.1002/pro.3735. Epub 2019 Oct 25. Protein Sci. 2019. PMID: 31583788 Free PMC article. Review.
Cited by
-
1-L Transcription in Prion Diseases.Int J Mol Sci. 2024 Sep 15;25(18):9961. doi: 10.3390/ijms25189961. Int J Mol Sci. 2024. PMID: 39337449 Free PMC article.
References
-
- Bremer J; Baumann F; Tiberi C; Wessig C; Fischer H; Schwarz P; Steele A; Toyka K; Nave K-A; Weis J et al. Axonal prion protein is required for peripheral myelin maintenance. Nat. Neurosci 2010, 13, 310–318. - PubMed
-
- Sáa P; Harris DA; Cervenakova L Mechanisms of prion-induced neurodegeneration. Expert Rev. Mol. Med 2016, 18, e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

