A Borane Lewis Acid in the Secondary Coordination Sphere of a Ni(II) Imido Imparts Distinct C-H Activation Selectivity
- PMID: 35973127
- PMCID: PMC10276360
- DOI: 10.1021/jacs.2c06662
A Borane Lewis Acid in the Secondary Coordination Sphere of a Ni(II) Imido Imparts Distinct C-H Activation Selectivity
Abstract
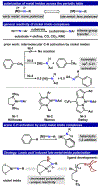
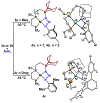
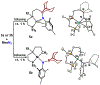
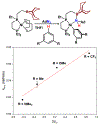
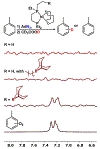
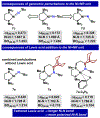
Two borane-functionalized bidentate phosphine ligands that vary in tether length have been prepared to examine cooperative metal-substrate interactions. Ni(0) complexes react with aryl azides at low temperatures to form structurally unusual κ2-(N,N)-N3Ar adducts. Warming these adducts affords products of N2 extrusion and in one case, a Ni-imido compound that is capped by the appended borane. Reactions with 1-azidoadamantane (AdN3) provide a distinct outcome, where a proposed nickel imido intermediate activates the sp2 C-H bonds of arenes, even in the presence of benzylic C-H sites. Combined experimental and computational mechanistic studies demonstrate that the unique reactivity is a consequence of Lewis-acid-induced polarization of the Ni-NR bond, potentially providing a synthetic strategy for chemoselective reaction engineering.
Figures




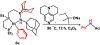
Similar articles
-
A Bidentate Ligand Featuring Ditopic Lewis Acids in the Second Sphere for Selective Substrate Capture and Activation.Angew Chem Int Ed Engl. 2023 Mar 20;62(13):e202218907. doi: 10.1002/anie.202218907. Epub 2023 Feb 20. Angew Chem Int Ed Engl. 2023. PMID: 36720708 Free PMC article.
-
Borylation and silylation of C-H bonds: a platform for diverse C-H bond functionalizations.Acc Chem Res. 2012 Jun 19;45(6):864-73. doi: 10.1021/ar200206a. Epub 2011 Nov 10. Acc Chem Res. 2012. PMID: 22075137
-
Mechanism and Selectivity Control in Ni- and Pd-Catalyzed Cross-Couplings Involving Carbon-Oxygen Bond Activation.Acc Chem Res. 2021 May 4;54(9):2158-2171. doi: 10.1021/acs.accounts.1c00050. Epub 2021 Apr 7. Acc Chem Res. 2021. PMID: 33826300
-
Transition metal-carboryne complexes: synthesis, bonding, and reactivity.Acc Chem Res. 2011 Apr 19;44(4):299-309. doi: 10.1021/ar100156f. Epub 2011 Mar 11. Acc Chem Res. 2011. PMID: 21395260 Review.
-
Dinickel Active Sites Supported by Redox-Active Ligands.Acc Chem Res. 2021 Oct 5;54(19):3710-3719. doi: 10.1021/acs.accounts.1c00424. Epub 2021 Sep 26. Acc Chem Res. 2021. PMID: 34565142 Free PMC article. Review.
Cited by
-
Hydrolase mimic via second coordination sphere engineering in metal-organic frameworks for environmental remediation.Nat Commun. 2023 Sep 25;14(1):5974. doi: 10.1038/s41467-023-41716-6. Nat Commun. 2023. PMID: 37749093 Free PMC article.
-
Bis(bicyclo[1.1.1]pentyl)chlorophosphine as a Precursor for the Preparation of Bis(bicyclo[1.1.1]pentyl)phosphines.Org Lett. 2024 Jul 26;26(29):6071-6075. doi: 10.1021/acs.orglett.4c01190. Epub 2024 May 12. Org Lett. 2024. PMID: 38735051 Free PMC article.
-
Facile Synthesis of Uranium Complexes with a Pendant Borane Lewis Acid and 1,2-Insertion of CO into a U-N Bond.Angew Chem Int Ed Engl. 2022 Dec 19;61(51):e202212823. doi: 10.1002/anie.202212823. Epub 2022 Nov 17. Angew Chem Int Ed Engl. 2022. PMID: 36256540 Free PMC article.
-
A Bidentate Ligand Featuring Ditopic Lewis Acids in the Second Sphere for Selective Substrate Capture and Activation.Angew Chem Int Ed Engl. 2023 Mar 20;62(13):e202218907. doi: 10.1002/anie.202218907. Epub 2023 Feb 20. Angew Chem Int Ed Engl. 2023. PMID: 36720708 Free PMC article.
-
Light-Driven Enantioselective Carbene-Catalyzed Radical-Radical Coupling.Angew Chem Int Ed Engl. 2023 Dec 4;62(49):e202312829. doi: 10.1002/anie.202312829. Epub 2023 Nov 6. Angew Chem Int Ed Engl. 2023. PMID: 37845183 Free PMC article.
References
-
- Zhao M; Wang HB; Ji LN; Mao ZW Insights into metalloenzyme microenvironments: biomimetic metal complexes with a functional second coordination sphere. Chem. Soc. Rev 2013, 42 (21), 8360–8375. - PubMed
-
- Elsby MR; Baker RT Strategies and mechanisms of metal–ligand cooperativity in first-row transition metal complex catalysts. Chem. Soc. Rev 2020, 49 (24), 8933–8987. - PubMed
-
- Trouve J; Gramage-Doria R Beyond hydrogen bonding: recent trends of outer sphere interactions in transition metal catalysis. Chem. Soc. Rev 2021, 50 (5), 3565–3584. - PubMed
-
- Drover MW A guide to secondary coordination sphere editing. Chem. Soc. Rev 2022, 51 (6), 1861–1880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

