Optimal Wireless Reflux Monitoring Metrics to Predict Discontinuation of Proton Pump Inhibitor Therapy
- PMID: 35973148
- PMCID: PMC9532366
- DOI: 10.14309/ajg.0000000000001871
Optimal Wireless Reflux Monitoring Metrics to Predict Discontinuation of Proton Pump Inhibitor Therapy
Abstract
Introduction: Ambulatory reflux monitoring performed off proton pump inhibitor (PPI) is the gold standard diagnostic test for nonerosive gastroesophageal reflux disease (GERD). However, the diagnostic metrics and optimal duration of monitoring are not well defined. This study evaluated the performance of multiple metrics across distinct durations of wireless reflux monitoring off PPI against the ability to discontinue PPI therapy in patients with suboptimal PPI response.
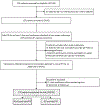
Methods: This single-arm clinical trial performed over 4 years at 2 centers enrolled adults with troublesome GERD symptoms and inadequate response to > 8 weeks of PPI. Participants underwent 96-hour wireless pH monitoring off PPI. Primary outcome was whether the subject successfully discontinued PPI or resumed PPI within 3 weeks.
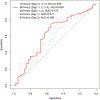
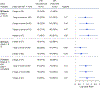
Results: Of 132 participants, 30% discontinued PPI. Among multiple metrics assessed, total acid exposure time (AET) of 4.0% performed best in predicting PPI discontinuation (odds ratio 2.9 [95% confidence interval 1.4, 6.4]; P = 0.006), with other thresholds of AET and DeMeester score performing comparably. AET was significantly higher on day 1 of monitoring compared with other days, and prognostic performance significantly declined when only assessing the first 48 hours of monitoring (area under the curve for 96 hours 0.63 vs area under the curve for 48 hours 0.57; P = 0.01).
Discussion: This clinical trial highlights the AET threshold of 4.0% as a high-performing prognostic marker of PPI discontinuation. 96 hours of monitoring performed better than 48 hours, in predicting ability to discontinue PPI. These data can inform current diagnostic approaches for patients with GERD symptoms who are unresponsive to PPI therapy.
Trial registration: ClinicalTrials.gov NCT03202537.
Copyright © 2022 by The American College of Gastroenterology.
Figures
References
-
- Zerbib F, Bredenoord AJ, Fass R, et al. ESNM/ANMS consensus paper: Diagnosis and management of refractory gastro-esophageal reflux disease. Neurogastroenterol Motil 2020:e14075. - PubMed
-
- Capovilla G, Salvador R, Spadotto L, et al. Long-term wireless pH monitoring of the distal esophagus: prolonging the test beyond 48 hours is unnecessary and may be misleading. Dis Esophagus 2017;30:1–8. - PubMed
-
- Roman S, Mion F, Zerbib F, et al. Wireless pH capsule--yield in clinical practice. Endoscopy 2012;44:270–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

