Linking the European Organisation for Research and Treatment of Cancer Item Library to the Common Terminology Criteria for Adverse Events
- PMID: 35973158
- PMCID: PMC9649281
- DOI: 10.1200/JCO.21.02017
Linking the European Organisation for Research and Treatment of Cancer Item Library to the Common Terminology Criteria for Adverse Events
Abstract
Purpose: The European Organisation for Research and Treatment of Cancer (EORTC) Item Library is an interactive online platform currently composed of 950 unique items (questions) derived from 67 patient-reported outcome (PRO) questionnaires. PROs complement clinician adverse event (AE) reporting classifications like the Common Terminology Criteria for Adverse Events (CTCAE). This work aims to create a standardized framework using the CTCAE to systematically classify symptomatic AEs from the EORTC Item Library through linking individual items to corresponding AEs.
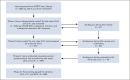
Methods: The EORTC Item Library items were searched for within the CTCAE (v5.0) and linked to an AE if they were described within the AE's title, description, or grading. Symptoms described in EORTC items but not located in the CTCAE were coded as missing symptoms. Other nonsymptom EORTC items, not described within the CTCAE were assigned a non-CTCAE descriptive classification. Further descriptive codes (eg, multiple issues) were allocated to enable descriptive analysis. Two raters independently coded 26.2% (n = 249) of the items. The remaining 701 items were coded by one rater and verified by the second, followed by discussion with two additional raters to reach consensus.
Results: Overall, 625 (65.8%) EORTC items were linked to 208 different AEs. Three hundred sixty-nine items provide information about non-CTCAE cancer-related issues and were categorized into seven descriptive classifications, including body image; emotional impact of a symptom, diagnosis, or treatment; global health and quality of life; and impact on life and daily activities. Inter-rater agreement for independent coding was 79.1%. Bowel urgency and tenesmus were identified as missing symptoms in CTCAEv5.0.
Conclusion: The EORTC Item Library provides considerable coverage of CTCAE toxicities, along with other complementary issues important to patients with cancer. Using the CTCAE clinical framework to classify symptomatic PRO items may facilitate PRO selection and use in clinical trials and routine care.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures
References
-
- U.S. Food and Drug Administration : FDA Oncology Center of Excellence Patient Focused Drug Development Program, 2020. https://www.fda.gov/about-fda/oncology-center-excellence/patient-focused...
-
- Kluetz PG, Kanapuru B, Lemery S, et al. : Informing the tolerability of cancer treatments using patient-reported outcome measures: Summary of an FDA and Critical Path Institute Workshop. Value Health 21:742-747, 2018 - PubMed
-
- National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v 5.0. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs...
-
- European Medicines Agency : Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man. The use of patient-reported outcome (PRO) measures in oncology studies, 2016. https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evalua...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

