The Effects of Modifying Amount and Type of Dietary Carbohydrate on Esophageal Acid Exposure Time and Esophageal Reflux Symptoms: A Randomized Controlled Trial
- PMID: 35973185
- PMCID: PMC9531994
- DOI: 10.14309/ajg.0000000000001889
The Effects of Modifying Amount and Type of Dietary Carbohydrate on Esophageal Acid Exposure Time and Esophageal Reflux Symptoms: A Randomized Controlled Trial
Abstract
Introduction: This is the first randomized controlled diet intervention trial to investigate both the amount and type of carbohydrate on symptomatic gastroesophageal reflux disease (GERD).
Methods: Ninety-eight veterans with symptomatic GERD were randomly assigned to high total/high simple, high total/low simple, low total/high simple, or low total/low simple carbohydrate diet for 9 weeks. The primary outcomes were esophageal acid exposure time (AET) and total number of reflux episodes derived from 24-hour ambulatory pH monitoring. Secondary outcomes were esophageal reflux symptoms rated using the Gastroesophageal Reflux Disease Questionnaire (GERDQ) and GERD Symptom Assessment Scale (GSAS).
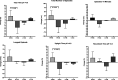
Results: Half of the subjects were White and half African American (mean age, 60.0 ± 12.5 years; mean body mass index, 32.7 ± 5.4 kg/m 2 ). There was a significant main effect of diet treatment on AET ( P = 0.001) and on the total number of reflux episodes ( P = 0.003). The change in AET in the high total/low simple group (-4.3% ± 3.8%) differed significantly from the high total/high simple control group (+3.1% ± 3.7%), (P = 0.04). The reduction in simple sugar intake averaged 62 g less per day. Subjects' ratings of symptoms improved in all carbohydrate modification groups, including significant reductions in heartburn frequency, heartburn severity, acid taste in the mouth, lump/pain in the throat or chest, and sleep disturbance.
Discussion: A modification of dietary carbohydrate intake that targeted a substantial reduction in the intakes of simple sugars improved pH monitoring outcomes and symptoms of GERD that profoundly affect daily life. These findings provide a feasible and clinically applicable contribution to the limited objective data existing for efficacious dietary recommendations in the routine treatment and management of GERD.
Trial registration: ClinicalTrials.gov NCT02384551.
Copyright © 2022 Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a "work of the United States Government" for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
Conflict of interest statement
Figures
References
-
- Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology 2002;122(5):1500–11. - PubMed
-
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108(3):308–28; quiz 329. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous