Safety and efficacy of classical complement pathway inhibition with sutimlimab in chronic immune thrombocytopenia
- PMID: 35973190
- PMCID: PMC10027504
- DOI: 10.1182/bloodadvances.2021006864
Safety and efficacy of classical complement pathway inhibition with sutimlimab in chronic immune thrombocytopenia
Abstract
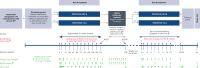
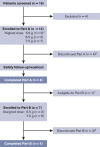
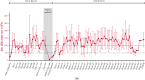
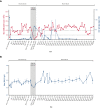
Chronic/refractory immune thrombocytopenia (ITP) is a rare and pathophysiologically heterogeneous disorder with variable responsiveness to available treatments. Sutimlimab, a first-in-class humanized monoclonal anti-C1s IgG4 antibody, selectively inhibits the classical pathway. This phase 1 study (NCT03275454) assessed the safety, efficacy, pharmacokinetics, and pharmacodynamics of biweekly sutimlimab in patients with chronic/refractory ITP with an inadequate response to ≥2 therapies (platelet count ≤ 30 × 109/L). Twelve patients (median age 42 years) received sutimlimab for a median of 20.5 weeks followed by a median 2-week washout period (part A). In part B, 7 of the 12 eligible patients received sutimlimab retreatment for a median of 113 weeks. In part A, the mean (standard deviation) platelet count increased from 25 × 109/L (17) to 54 × 109/L (60) 24 hours after starting sutimlimab, maintaining ≥50 × 109/L throughout part A. Five patients (42%) achieved durable platelet count responses (≥50 × 109/L in ≥50% of follow-up visits) and 4 achieved complete response (platelet count ≥100 × 109/L). The mean platelet count returned to baseline during washout and increased upon retreatment in part B. The mean platelet count improvements accompanied the rapid inhibition of the classical pathway. There were 74 treatment-emergent adverse events in part A (n = 10) and 70 in part B (n = 6). Five serious adverse events were observed; 1 event (migraine) was assessed by the investigator as related to sutimlimab. These results demonstrated that in some patients with ITP, autoantibodies activate the classical complement pathway, accelerating platelet destruction or impairing platelet production and contributing to treatment failure. Thus, C1s inhibition may be a safe and beneficial therapeutic approach for patients with chronic/refractory ITP.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.M.B., Alexion Pharmaceuticals, Inc: honoraria, research funding; Bioverativ: honoraria, research funding; Cellphire: honoraria, research funding; Incyte: honoraria, research funding; Rigel: honoraria, research funding; Sanofi Genzyme: honoraria, research funding. A.R., Sanofi: consultancy, honoraria; Roche: consultancy, honoraria, research funding; Novartis: consultancy, honoraria; Biocryst: consultancy, honoraria; Apellis: consultancy, honoraria; Alexion Pharmaceuticals, Inc: consultancy, honoraria, research funding. D.J.K., UpToDate: consultancy, honoraria, patents, royalties; Shionogi: consultancy, honoraria; Momenta: consultancy, honoraria; Merck Sharp & Dohme: consultancy, honoraria; Kyowa-Kirin: consultancy, honoraria; Kezar Life Sciences, Inc: other, research funding; CRICO: consultancy, honoraria; Caremark: consultancy, honoraria; Immunovant: other: travel expenses, research funding; Bristol-Myers Squibb: consultancy, honoraria, other: travel expenses, research funding; Argenx: consultancy, honoraria, other: travel expenses, research funding; Sanofi (Genzyme): consultancy, honoraria; Zafgen: consultancy, honoraria; Protalex: consultancy, honoraria, research funding; Shionogi: consultancy; Protalix Biotherapeutics: consultancy; Shire: consultancy, honoraria; Principia: consultancy, research funding; Dova: consultancy, honoraria; Takeda: consultancy, honoraria, other, research funding; Rigel: consultancy, honoraria, other, research funding; Protalex: consultancy, honoraria, other, research funding; Principia Biopharma: consultancy, honoraria, other, research funding; Incyte: consultancy, honoraria; Immunovant: consultancy, honoraria; Genzyme: consultancy, honoraria; Amgen: consultancy, honoraria, other: travel expenses, research funding; Alnylam: consultancy, honoraria, other: travel expenses, research funding; Agios: consultancy, honoraria, other: travel expenses, research funding; Daiichi Sankyo: consultancy, honoraria; Actelion (Syntimmune): consultancy, honoraria, other: travel expenses, research funding; UCB: consultancy, honoraria; Platelet Disorder Support Association: consultancy, honoraria; Pfizer: consultancy, honoraria; Novartis: consultancy, honoraria. M.S., Takeda: consultancy, speakers bureau; Takeda: speakers bureau; Novartis: other: advisory board, speakers bureau; Alexion: consultancy, speakers bureau; Shire/Takeda: other: advisory board, research funding, speakers bureau; Ablynx/Sanofi: consultancy, other: advisory board, speakers bureau; Sanofi: consultancy, speakers bureau. R.S., Bioverativ: honoraria; Alnylam: honoraria. J.W., Sanofi: current employment. C.R., Sanofi: employment at the time of the study (ended employment in the past 24 months). W.H., Sanofi: employment at the time of the study (ended employment in the past 24 months). A.D., Sanofi: current employment, current equity holder in publicly traded company.
Figures


References
-
- Feudjo-Tepie MA, Robinson NJ, Bennett D. Prevalence of diagnosed chronic immune thrombocytopenic purpura in the US: analysis of a large US claim database: a rebuttal. J Thromb Haemost. 2008;6(4):711–712. author reply 713. - PubMed
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. - PubMed
-
- Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP) J Clin Med. 2017;6(2):16. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

