Continued treatment with nintedanib in patients with systemic sclerosis-associated interstitial lung disease: data from SENSCIS-ON
- PMID: 35973804
- PMCID: PMC9664126
- DOI: 10.1136/ard-2022-222564
Continued treatment with nintedanib in patients with systemic sclerosis-associated interstitial lung disease: data from SENSCIS-ON
Abstract
Objectives: In the SENSCIS trial in patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD), nintedanib reduced the rate of decline in forced vital capacity (FVC) versus placebo, with adverse events that were manageable for most patients. An open-label extension trial, SENSCIS-ON, is assessing safety and FVC decline during longer term nintedanib treatment.
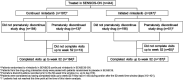
Methods: Patients who completed the SENSCIS trial or a drug-drug interaction (DDI) study of nintedanib and oral contraceptive on treatment were eligible to enter SENSCIS-ON. Adverse events and changes in FVC over 52 weeks of SENSCIS-ON were assessed in patients who received nintedanib in SENSCIS and continued nintedanib in SENSCIS-ON ('continued nintedanib' group) and in patients who received placebo in SENSCIS and initiated nintedanib in SENSCIS-ON or who received nintedanib for ≤28 days in the DDI study ('initiated nintedanib' group).
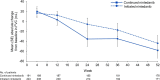
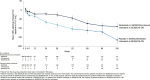
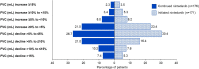
Results: There were 197 patients in the continued nintedanib group and 247 in the initiated nintedanib group. Diarrhoea was reported in 68.0% and 68.8% of patients in these groups, respectively. Adverse events led to discontinuation of nintedanib in 4.6% and 21.5% of the continued nintedanib and initiated nintedanib groups, respectively. Mean (SE) changes in FVC from baseline to week 52 of SENSCIS-ON were -58.3 (15.5) mL in the continued nintedanib group and -44.0 (16.2) mL in the initiated nintedanib group.
Conclusions: The safety profile of nintedanib over 52 weeks of SENSCIS-ON was consistent with that reported in SENSCIS. The change in FVC over 52 weeks of SENSCIS-ON was similar to that observed in the nintedanib group of SENSCIS.
Keywords: Autoimmune Diseases; Pulmonary Fibrosis; Scleroderma, Systemic.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Yannick Allanore has received consulting fees from Boehringer Ingelheim (BI) and Sanofi; speaking fees from AbbVie, BI, Janssen; and has participated on Data Safety Monitoring Boards or Advisory Boards for BI, Chemomab, Curzion, Medsenic, Menarini, Prometheus, Sanofi. Madelon C Vonk reports grants from BI, Ferrer, Galapagos, Janssen; speaking fees from BI, Janssen, Merck Sharp & Dohme; has participated on a Data Safety Monitoring Board or Advisory Board for Corbus; and has an unpaid role with EUSTAR. Oliver Distler has received grants from BI, Kymera, Mitsubishi Tanabe; consultancy fees from AbbVie, Acceleron Pharma, Alcimed, Amgen, AnaMar, Arxx Therapeutics, AstraZeneca, Bayer, Beacon, Blade Therapeutics, BI, Corbus Pharmaceuticals, CSL Behring, 4P Science, Galapagos, Glenmark Pharmaceuticals, Horizon Therapeutics (Curzion), Inventiva, Kymera, Lupin, Merck Sharp & Dohme, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Pfizer, Prometheus Biosciences, Roivant Sciences, Sanofi, Topadur; speaking fees from Bayer, BI, Janssen, Medscape; and holds patent US8247389 for the treatment of SSc; he is Chair of the Executive Committee for the FOREUM Foundation, Co-Chair of the ERS/EULAR Guidelines, President of EUSTAR, a Member of the Board of Trustees for the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) and the Hartmann Müller Foundation, and a Senate member of the Swiss Academy of Medical Sciences (SAMW). Arata Azuma reports grants from BI; consulting fees from BI, Kyorin, Taiho, Toray; speaking fees from BI. Maureen D Mayes reports grants paid to her institution from BI, Corbus, Eicos, Horizon Therapeutics, Mitsubishi Tanabe; payment for acting as a grant reviewer from Actelion; speaking fees from Medtelligence; and has participated on Data Safety Monitoring Boards or Advisory Boards for BI, Eicos, Mitsubishi Tanabe. Martina Gahlemann, Veronika Kohlbrenner and Margarida Alves are employees of BI. Alexandra James is an employee of Elderbrook Solutions GmbH, which was contracted by BI to conduct the analyses presented in this manuscript. Dinesh Khanna reports grants from Bristol Myers Squibb, Horizon Therapeutics, Pfizer; consulting fees from AbbVie, Actelion, BI, Bristol Myers Squibb, CSL Behring, Genentech, Horizon Therapeutics, Talaris, Theraly; speaking fees from AbbVie, Actelion, BI, CSL Behring, Genentech, Horizon Therapeutics; has a leadership or fiduciary role with Eicos; has received royalties or licenses for the University of California Los Angeles Scleroderma Clinical Trials Consortium Gastrointestinal Tract 2.0; and owns stock in Eicos. Kristin B Highland reports grants paid to her institution from BI; consulting and speaking fees from BI; and is a Scientific Advisory Committee Member for the National Scleroderma Foundation in the USA.
Figures

Similar articles
-
Continued nintedanib in patients with systemic sclerosis-associated interstitial lung disease: 3-year data from SENSCIS-ON.RMD Open. 2025 Feb 23;11(1):e005086. doi: 10.1136/rmdopen-2024-005086. RMD Open. 2025. PMID: 39988350 Free PMC article. Clinical Trial.
-
Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial.Lancet Respir Med. 2021 Jan;9(1):96-106. doi: 10.1016/S2213-2600(20)30330-1. Lancet Respir Med. 2021. PMID: 33412120 Clinical Trial.
-
Trajectories of forced vital capacity in patients with systemic sclerosis-associated interstitial lung disease.Arthritis Res Ther. 2025 Mar 21;27(1):63. doi: 10.1186/s13075-025-03524-9. Arthritis Res Ther. 2025. PMID: 40119463 Free PMC article. Clinical Trial.
-
Interstitial lung disease in patients with systemic sclerosis: what can we learn from the SENSCIS trial?Clin Exp Rheumatol. 2023 Aug;41(8):1713-1719. doi: 10.55563/clinexprheumatol/trcv91. Epub 2023 Aug 3. Clin Exp Rheumatol. 2023. PMID: 37534955 Review.
-
Nintedanib: A Review in Fibrotic Interstitial Lung Diseases.Drugs. 2021 Apr;81(5):575-586. doi: 10.1007/s40265-021-01487-0. Epub 2021 Mar 25. Drugs. 2021. PMID: 33765296 Free PMC article. Review.
Cited by
-
Nintedanib Therapy Alone and Combined with Mycophenolate in Patients with Systemic Sclerosis-associated Interstitial Lung Disease: Systematic Reviews and Meta-analysis.Ann Am Thorac Soc. 2024 Mar;21(3):474-485. doi: 10.1513/AnnalsATS.202301-081OC. Ann Am Thorac Soc. 2024. PMID: 37773000 Free PMC article.
-
Treatment of Systemic Sclerosis-associated Interstitial Lung Disease: Evidence-based Recommendations. An Official American Thoracic Society Clinical Practice Guideline.Am J Respir Crit Care Med. 2024 Jan 15;209(2):137-152. doi: 10.1164/rccm.202306-1113ST. Am J Respir Crit Care Med. 2024. PMID: 37772985 Free PMC article.
-
Continued nintedanib in patients with systemic sclerosis-associated interstitial lung disease: 3-year data from SENSCIS-ON.RMD Open. 2025 Feb 23;11(1):e005086. doi: 10.1136/rmdopen-2024-005086. RMD Open. 2025. PMID: 39988350 Free PMC article. Clinical Trial.
-
Nintedanib in Idiopathic Pulmonary Fibrosis: Tolerability and Safety in a Real Life Experience in a Single Centre in Patients also Treated with Oral Anticoagulant Therapy.Pharmaceuticals (Basel). 2023 Feb 16;16(2):307. doi: 10.3390/ph16020307. Pharmaceuticals (Basel). 2023. PMID: 37259452 Free PMC article.
-
Learnings from clinical trials in patients with connective tissue disease-associated interstitial lung disease.Arthritis Res Ther. 2023 Jul 8;25(1):118. doi: 10.1186/s13075-023-03090-y. Arthritis Res Ther. 2023. PMID: 37422652 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

