A TrkB agonist prodrug prevents bone loss via inhibiting asparagine endopeptidase and increasing osteoprotegerin
- PMID: 35973996
- PMCID: PMC9381595
- DOI: 10.1038/s41467-022-32435-5
A TrkB agonist prodrug prevents bone loss via inhibiting asparagine endopeptidase and increasing osteoprotegerin
Abstract
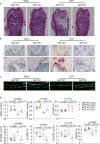
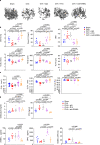
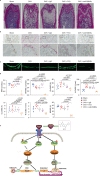
Brain-derived neurotrophic factor (BDNF) and its tropomyosin-related kinase B receptor (TrkB) are expressed in human osteoblasts and mediate fracture healing. BDNF/TrkB signaling activates Akt that phosphorylates and inhibits asparagine endopeptidase (AEP), which regulates the differentiation fate of human bone marrow stromal cells (hBMSC) and is altered in postmenopausal osteoporosis. Here we show that R13, a small molecular TrkB receptor agonist prodrug, inhibits AEP and promotes bone formation. Though both receptor activator of nuclear factor kappa-Β ligand (RANK-L) and osteoprotegerin (OPG) induced by ovariectomy (OVX) remain comparable between WT and BDNF+/- mice, R13 treatment significantly elevates OPG in both mice without altering RANKL, blocking trabecular bone loss. Strikingly, both R13 and anti-RANK-L exhibit equivalent therapeutic efficacy. Moreover, OVX increases RANK-L and OPG in WT and AEP KO mice with RANK-L/OPG ratio lower in the latter than the former, attenuating bone turnover. 7,8-DHF, released from R13, activates TrkB and its downstream effector CREB, which is critical for OPG augmentation. Consequently, 7,8-DHF represses C/EBPβ/AEP pathway, inhibiting RANK-L-induced RAW264.7 osteoclastogenesis. Therefore, our findings support that R13 exerts its therapeutic efficacy toward osteoporosis via inhibiting AEP and escalating OPG.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Gene silencing of the BDNF/TrkB axis in multiple myeloma blocks bone destruction and tumor burden in vitro and in vivo.Int J Cancer. 2013 Sep 1;133(5):1074-84. doi: 10.1002/ijc.28116. Epub 2013 Mar 8. Int J Cancer. 2013. PMID: 23420490
-
Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system.Endocrinology. 2005 Apr;146(4):1991-8. doi: 10.1210/en.2004-1167. Epub 2004 Dec 23. Endocrinology. 2005. PMID: 15618359
-
The RANKL/RANK/OPG pathway.Curr Osteoporos Rep. 2007 Sep;5(3):98-104. doi: 10.1007/s11914-007-0024-y. Curr Osteoporos Rep. 2007. PMID: 17925190
-
[Osteoprotegerin--a neutralizing receptor, protector of bones and a potential antiresorptive agent].Med Pregl. 2005 Jul-Aug;58(7-8):362-7. doi: 10.2298/mpns0508362a. Med Pregl. 2005. PMID: 16296579 Review. Serbian.
-
RANK-Fc: a therapeutic antagonist for RANK-L in myeloma.Cancer. 2003 Feb 1;97(3 Suppl):802-12. doi: 10.1002/cncr.11134. Cancer. 2003. PMID: 12548579 Review.
Cited by
-
Do Not Lose Your Nerve, Be Callus: Insights Into Neural Regulation of Fracture Healing.Curr Osteoporos Rep. 2024 Feb;22(1):182-192. doi: 10.1007/s11914-023-00850-2. Epub 2024 Jan 31. Curr Osteoporos Rep. 2024. PMID: 38294715 Free PMC article. Review.
-
Targeting the central and peripheral nervous system to regulate bone homeostasis: mechanisms and potential therapies.Mil Med Res. 2025 Mar 20;12(1):13. doi: 10.1186/s40779-025-00600-8. Mil Med Res. 2025. PMID: 40108680 Free PMC article. Review.
-
Brain-Derived Neurotrophic Factor (BDNF) Enhances Osteogenesis and May Improve Bone Microarchitecture in an Ovariectomized Rat Model.Cells. 2024 Mar 15;13(6):518. doi: 10.3390/cells13060518. Cells. 2024. PMID: 38534361 Free PMC article.
-
Alzheimer's Disease and Impaired Bone Microarchitecture, Regeneration and Potential Genetic Links.Life (Basel). 2023 Jan 29;13(2):373. doi: 10.3390/life13020373. Life (Basel). 2023. PMID: 36836731 Free PMC article. Review.
-
Liraglutide attenuates palmitate-induced apoptosis via PKA/β-catenin/Bcl-2/Bax pathway in MC3T3-E1 cells.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jan;397(1):329-341. doi: 10.1007/s00210-023-02572-9. Epub 2023 Jul 13. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37439807
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

