The addition of FAIMS increases targeted proteomics sensitivity from FFPE tumor biopsies
- PMID: 35974054
- PMCID: PMC9381555
- DOI: 10.1038/s41598-022-16358-1
The addition of FAIMS increases targeted proteomics sensitivity from FFPE tumor biopsies
Abstract
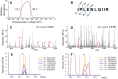
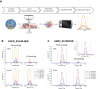
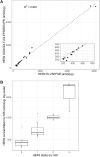
Mass spectrometry-based targeted proteomics allows objective protein quantitation of clinical biomarkers from a single section of formalin-fixed, paraffin-embedded (FFPE) tumor tissue biopsies. We combined high-field asymmetric waveform ion mobility spectrometry (FAIMS) and parallel reaction monitoring (PRM) to increase assay sensitivity. The modular nature of the FAIMS source allowed direct comparison of the performance of FAIMS-PRM to PRM. Limits of quantitation were determined by spiking synthetic peptides into a human spleen matrix. In addition, 20 clinical samples were analyzed using FAIMS-PRM and the quantitation of HER2 was compared with that obtained with the Ventana immunohistochemistry assay. FAIMS-PRM improved the overall signal-to-noise ratio over that from PRM and increased assay sensitivity in FFPE tissue analysis for four (HER2, EGFR, cMET, and KRAS) of five proteins of clinical interest. FAIMS-PRM enabled sensitive quantitation of basal HER2 expression in breast cancer samples classified as HER2 negative by immunohistochemistry. Furthermore, we determined the degree of FAIMS-dependent background reduction and showed that this correlated with an improved lower limit of quantitation with FAIMS. FAIMS-PRM is anticipated to benefit clinical trials in which multiple biomarker questions must be addressed and the availability of tumor biopsy samples is limited.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- U.S. Food and Drug Administration List of cleared or approved companion diagnostic devices (in vitro and imaging tools). https://www.fda.gov/media/119249/download (March 24, 2021),
-
- Scheerens H, Malong A, Bassett K, Boyd Z, Gupta V, Harris J, Mesick C, Simnett S, Stevens H, Gilbert H, Risser P, Kalamegham R, Jordan J, Engel J, Chen S, Essioux L, Williams JA. Current status of companion and complementary diagnostics: Strategic considerations for development and launch. Clin. Transl. Sci. 2017;10(2):84–92. doi: 10.1111/cts.12455. - DOI - PMC - PubMed
-
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. - DOI - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

