Application of immune enhanced organoids in modeling personalized Merkel cell carcinoma research
- PMID: 35974123
- PMCID: PMC9380677
- DOI: 10.1038/s41598-022-17921-6
Application of immune enhanced organoids in modeling personalized Merkel cell carcinoma research
Abstract
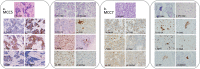
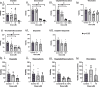
Merkel cell carcinoma (MCC) is a rare neuroendocrine cutaneous cancer, with incidence of less than 1/100,000, low survival rates and variable response to chemotherapy or immunotherapy. Herein we explore the application of patient tumor organoids (PTOs) in modeling personalized research in this rare malignancy. Unsorted and non-expanded MCC tumor cells were isolated from surgical specimens and suspended in an ECM based hydrogel, along with patient matched blood and lymph node tissue to generate immune enhanced organoids (iPTOs). Organoids were treated with chemotherapy or immunotherapy agents and efficacy was determined by post-treatment viability. Nine specimens from seven patients were recruited from December 2018-January 2022. Establishment rate was 88.8% (8/9) for PTOs and 77.8% (7/9) for iPTOs. Histology on matched patient tissues and PTOs demonstrated expression of MCC markers. Chemotherapy response was exhibited in 4/6 (66.6%) specimens with cisplatin and doxorubicin as the most effective agents (4/6 PTO sets) while immunotherapy was not effective in tested iPTO sets. Four specimens from two patients demonstrated resistance to pembrolizumab, correlating with the corresponding patient's treatment response. Routine establishment and immune enhancement of MCC PTOs is feasible directly from resected surgical specimens allowing for personalized research and exploration of treatment regimens in the preclinical setting.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

