Tuning immunity through tissue mechanotransduction
- PMID: 35974148
- PMCID: PMC9379893
- DOI: 10.1038/s41577-022-00761-w
Tuning immunity through tissue mechanotransduction
Abstract
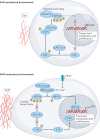
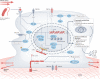
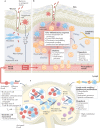
Immune responses are governed by signals from the tissue microenvironment, and in addition to biochemical signals, mechanical cues and forces arising from the tissue, its extracellular matrix and its constituent cells shape immune cell function. Indeed, changes in biophysical properties of tissue alter the mechanical signals experienced by cells in many disease conditions, in inflammatory states and in the context of ageing. These mechanical cues are converted into biochemical signals through the process of mechanotransduction, and multiple pathways of mechanotransduction have been identified in immune cells. Such pathways impact important cellular functions including cell activation, cytokine production, metabolism, proliferation and trafficking. Changes in tissue mechanics may also represent a new form of 'danger signal' that alerts the innate and adaptive immune systems to the possibility of injury or infection. Tissue mechanics can change temporally during an infection or inflammatory response, offering a novel layer of dynamic immune regulation. Here, we review the emerging field of mechanoimmunology, focusing on how mechanical cues at the scale of the tissue environment regulate immune cell behaviours to initiate, propagate and resolve the immune response.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures