Comparative biomarker analysis of PALOMA-2/3 trials for palbociclib
- PMID: 35974168
- PMCID: PMC9381541
- DOI: 10.1038/s41698-022-00297-1
Comparative biomarker analysis of PALOMA-2/3 trials for palbociclib
Abstract
While cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, including palbociclib, combined with endocrine therapy (ET), are becoming the standard-of-care for hormone receptor-positive/human epidermal growth factor receptor 2‒negative metastatic breast cancer, further mechanistic insights are needed to maximize benefit from the treatment regimen. Herein, we conducted a systematic comparative analysis of gene expression/progression-free survival relationship from two phase 3 trials (PALOMA-2 [first-line] and PALOMA-3 [≥second-line]). In the ET-only arm, there was no inter-therapy line correlation. However, adding palbociclib resulted in concordant biomarkers independent of initial ET responsiveness, with shared sensitivity genes enriched in estrogen response and resistance genes over-represented by mTORC1 signaling and G2/M checkpoint. Biomarker patterns from the combination arm resembled patterns observed in ET in advanced treatment-naive patients, especially patients likely to be endocrine-responsive. Our findings suggest palbociclib may recondition endocrine-resistant tumors to ET, and may guide optimal therapeutic sequencing by partnering CDK4/6 inhibitors with different ETs. Pfizer (NCT01740427; NCT01942135).
© 2022. The Author(s).
Conflict of interest statement
N.C.T. has been a consultant or advisor for Pfizer Inc; and has received research funding from Pfizer Inc, Eli Lilly, and Novartis. S.L. has received research funding from Merck, Novartis, Roche, and Genentech. F.A. has received research funding from AstraZeneca, Novartis, Pfizer Inc, Eli Lilly, and Daiichi Sankyo, and served as a speaker/advisor for AstraZeneca, Novartis, Pfizer Inc, Eli Lilly, and Daiichi Sankyo (compensated to his institution). M.M. has received research grants from Roche and Novartis and speakers honoraria from AstraZeneca, Amgen, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, and Pfizer Inc. V.D., has received consulting fees from Genentech, Pfizer Inc, AbbVie, Novartis Pharma KK, Roche-Peru, Eli Lilly, AstraZeneca, and Daiichi and serves on speakers bureaus for Pfizer Inc, Novartis Pharma KK, Roche-Peru, and AstraZeneca. K.A.G. has received consulting/advisory fees from Pfizer Inc, Novartis, AstraZeneca, Roche, Eli Lilly, Mylan, Bristol-Myers Squibb, NanoString Technologies, and Merck. N.H., has been a consultant or advisor for Eli Lilly, Novartis, and Pfizer Inc. H.S.R. has received research grant/funding from Pfizer Inc, Merck, Novartis, Lilly, Genentech, Odonate, Daiichi, Seattle Genetics, Eisai, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, and Immunomedics; and has received honoraria from PUMA, Samsung, and Mylan. S.L. reports research fees and to her institution from AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Cepheid, Eirgenix, GlaxoSmithKline, Immunomedics, Eli Lilly, Merck/MSD, Merck Kg, Novartis, Pfizer Inc, Pierre-Fabre, Puma, Roche, Samsung, Teva, and Vifor. M.C. has received consulting fees from Novartis, Merus, CytoDyn, Sermonix, and G1 Therapeutics; honoraria from Pfizer Inc; and research funding from Pfizer Inc, Novartis, Merus, Lilly, and G1 Therapeutics. R.S. F. has received consulting fees from Pfizer Inc, AstraZeneca, Bayer, Novartis, Bristol-Myers Squibb, Eisai, Eli Lilly, Merck, and Roche, as well as other research funding from Pfizer Inc and honoraria from Bayer, Pfizer Inc, Bristol-Myers Squibb, Novartis, Eisai, and Eli Lilly. Z.Z., C.Z., J.Q.C., Z.Y., D.R.L., P.W., T.L.V., P.A.R., X.H. and Y.L. are or were employees of and own stock in Pfizer Inc.
Figures
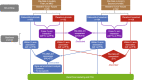


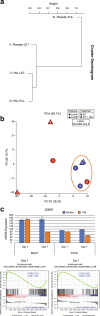
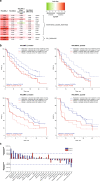
Similar articles
-
Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in patients with bone-only disease: a joint analysis of PALOMA-2 and PALOMA-3 clinical trials.Breast Cancer Res Treat. 2020 Nov;184(1):23-35. doi: 10.1007/s10549-020-05782-4. Epub 2020 Aug 11. Breast Cancer Res Treat. 2020. PMID: 32783178 Free PMC article. Clinical Trial.
-
Neutropenia management with palbociclib in Japanese patients with advanced breast cancer.Breast Cancer. 2019 Sep;26(5):637-650. doi: 10.1007/s12282-019-00970-7. Epub 2019 May 24. Breast Cancer. 2019. PMID: 31127500 Free PMC article. Clinical Trial.
-
Palbociclib Combined with Fulvestrant in Premenopausal Women with Advanced Breast Cancer and Prior Progression on Endocrine Therapy: PALOMA-3 Results.Oncologist. 2017 Sep;22(9):1028-1038. doi: 10.1634/theoncologist.2017-0072. Epub 2017 Jun 26. Oncologist. 2017. PMID: 28652278 Free PMC article. Clinical Trial.
-
A current and comprehensive review of cyclin-dependent kinase inhibitors for the treatment of metastatic breast cancer.Curr Med Res Opin. 2017 Sep;33(9):1559-1569. doi: 10.1080/03007995.2017.1348344. Epub 2017 Jul 25. Curr Med Res Opin. 2017. PMID: 28657360 Review.
-
Palbociclib: A Novel Cyclin-Dependent Kinase Inhibitor for Hormone Receptor-Positive Advanced Breast Cancer.Ann Pharmacother. 2015 Nov;49(11):1252-60. doi: 10.1177/1060028015602273. Epub 2015 Aug 31. Ann Pharmacother. 2015. PMID: 26324355 Free PMC article. Review.
Cited by
-
Understanding and overcoming CDK4/6 inhibitor resistance in HR+/HER2- metastatic breast cancer: clinical and molecular perspectives.Ther Adv Med Oncol. 2025 Jul 10;17:17588359251353623. doi: 10.1177/17588359251353623. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40656600 Free PMC article. Review.
-
Cancer takes many paths through G1/S.Trends Cell Biol. 2024 Aug;34(8):636-645. doi: 10.1016/j.tcb.2023.10.007. Epub 2023 Nov 10. Trends Cell Biol. 2024. PMID: 37953123 Free PMC article. Review.
-
Neratinib enhances the efficacy of CDK4/6 inhibitor plus endocrine therapy in HR+/HER2-low breast cancer cell line ZR-75-1 via hsa-miR-23a-5p.Sci Rep. 2024 Dec 28;14(1):31062. doi: 10.1038/s41598-024-82137-9. Sci Rep. 2024. PMID: 39730704 Free PMC article.
-
Glutaminase as a metabolic target of choice to counter acquired resistance to Palbociclib by colorectal cancer cells.Oncogene. 2025 Sep;44(36):3386-3406. doi: 10.1038/s41388-025-03495-w. Epub 2025 Jul 22. Oncogene. 2025. PMID: 40696167 Free PMC article.
-
Overall survival in Japanese patients with ER+/HER2- advanced breast cancer treated with first-line palbociclib plus letrozole.Breast Cancer. 2024 Jan;31(1):53-62. doi: 10.1007/s12282-023-01511-z. Epub 2023 Oct 26. Breast Cancer. 2024. PMID: 37882974 Free PMC article.
References
Associated data
Grants and funding
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
- N/A/Pfizer (Pfizer Inc.)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

