AIF Overexpression Aggravates Oxidative Stress in Neonatal Male Mice After Hypoxia-Ischemia Injury
- PMID: 35974295
- PMCID: PMC9525408
- DOI: 10.1007/s12035-022-02987-0
AIF Overexpression Aggravates Oxidative Stress in Neonatal Male Mice After Hypoxia-Ischemia Injury
Abstract
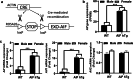
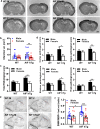
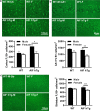
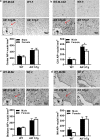
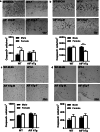
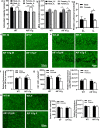
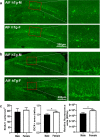
There are sex differences in the severity, mechanisms, and outcomes of neonatal hypoxia-ischemia (HI) brain injury, and apoptosis-inducing factor (AIF) may play a critical role in this discrepancy. Based on previous findings that AIF overexpression aggravates neonatal HI brain injury, we further investigated potential sex differences in the severity and molecular mechanisms underlying the injury using mice that overexpress AIF from homozygous transgenes. We found that the male sex significantly aggravated AIF-driven brain damage, as indicated by the injury volume in the gray matter (2.25 times greater in males) and by the lost volume of subcortical white matter (1.71 greater in males) after HI. As compared to females, male mice exhibited more severe brain injury, correlating with reduced antioxidant capacities, more pronounced protein carbonylation and nitration, and increased neuronal cell death. Under physiological conditions (without HI), the doublecortin-positive area in the dentate gyrus of females was 1.15 times larger than in males, indicating that AIF upregulation effectively promoted neurogenesis in females in the long term. We also found that AIF stimulated carbohydrate metabolism in young males. Altogether, these findings corroborate earlier studies and further demonstrate that AIF is involved in oxidative stress, which contributes to the sex-specific differences observed in neonatal HI brain injury.
Keywords: Apoptosis; Apoptosis-inducing factor; Hypoxia ischemia; Neonate; Oxidative stress; Sex difference.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Overexpression of apoptosis inducing factor aggravates hypoxic-ischemic brain injury in neonatal mice.Cell Death Dis. 2020 Jan 30;11(1):77. doi: 10.1038/s41419-020-2280-z. Cell Death Dis. 2020. PMID: 32001673 Free PMC article.
-
Inhibiting the interaction between apoptosis-inducing factor and cyclophilin A prevents brain injury in neonatal mice after hypoxia-ischemia.Neuropharmacology. 2020 Jul;171:108088. doi: 10.1016/j.neuropharm.2020.108088. Epub 2020 Apr 8. Neuropharmacology. 2020. PMID: 32277944
-
Lack of the brain-specific isoform of apoptosis-inducing factor aggravates cerebral damage in a model of neonatal hypoxia-ischemia.Cell Death Dis. 2018 Dec 18;10(1):3. doi: 10.1038/s41419-018-1250-1. Cell Death Dis. 2018. PMID: 30584234 Free PMC article.
-
Apoptosis-inducing factor downregulation increased neuronal progenitor, but not stem cell, survival in the neonatal hippocampus after cerebral hypoxia-ischemia.Mol Neurodegener. 2012 Apr 25;7:17. doi: 10.1186/1750-1326-7-17. Mol Neurodegener. 2012. PMID: 22534064 Free PMC article.
-
Impact of peripheral immune cells in experimental neonatal hypoxia-ischemia: A systematic review and meta-analysis.Int Immunopharmacol. 2025 Jan 3;145:113682. doi: 10.1016/j.intimp.2024.113682. Epub 2024 Dec 4. Int Immunopharmacol. 2025. PMID: 39637576
Cited by
-
Hypoxia-ischemia and sexual dimorphism: modeling mitochondrial dysfunction using brain organoids.Cell Biosci. 2025 May 24;15(1):67. doi: 10.1186/s13578-025-01402-0. Cell Biosci. 2025. PMID: 40413513 Free PMC article. Review.
-
TrkB-mediated sustained neuroprotection is sex-specific and Erα-dependent in adult mice following neonatal hypoxia ischemia.Biol Sex Differ. 2024 Jan 4;15(1):1. doi: 10.1186/s13293-023-00573-0. Biol Sex Differ. 2024. PMID: 38178264 Free PMC article.
References
-
- Sequeira A, Gil J, Sampaio I, Moniz C, Graca A. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy: 10-year experience. Revista Portuguesa de Pediatria. 2021;52:21–29. doi: 10.25754/pjp.2021.20006. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

