The Efficacy and Safety of New-Generation Intense Pulsed Light in the Treatment of Meibomian Gland Dysfunction-Related Dry Eye: A Multicenter, Randomized, Patients-Blind, Parallel-Control, Non-Inferiority Clinical Trial
- PMID: 35974296
- PMCID: PMC9437192
- DOI: 10.1007/s40123-022-00556-1
The Efficacy and Safety of New-Generation Intense Pulsed Light in the Treatment of Meibomian Gland Dysfunction-Related Dry Eye: A Multicenter, Randomized, Patients-Blind, Parallel-Control, Non-Inferiority Clinical Trial
Abstract
Introduction: This study aimed to evaluate the efficacy and safety of a new-generation intense pulsed light (IPL) device in improving the symptoms and signs of meibomian gland dysfunction (MGD)-related dry eye, and compare it with a traditional IPL device.
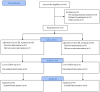
Methods: This multicenter randomized controlled trial enrolled 132 patients with MGD-related dry eye from two centers. Patients were randomly assigned into the new-generation IPL (Eyesis) group or traditional IPL (E-Eye) group, and then blinded to receive treatment on days 0 and 7. Ocular Surface Disease Index (OSDI), tear meniscus height (TMH), tear breakup time (TBUT), corneal fluorescein staining (CFS), Schirmer test, and meibomian gland signs were evaluated on days 0, 7, and 14. The primary outcome was defined as the effective rate of treating MGD at day 14. Any adverse events were recorded for safety assessment. Intergroup comparisons and non-inferiority analysis were performed. p values less than 0.05 were considered statistically significant.
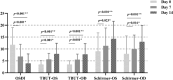
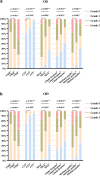
Results: Basic information showed no significant difference between treatment groups. The intergroup difference of the effective rate was - 1.7% in the left eye and 1.6% in right eye, verifying the non-inferiority of the Eyesis device (p = 0.927). Significant improvements in OSDI, TBUT, Schirmer test, TMH, CFS, and meibomian gland signs were observed in Eyesis group on days 7 and 14 (all p < 0.05). Compared to the E-Eye group, the Eyesis group achieved more significant improvements in OSDI, TBUT, Schirmer test, TMH, and meibum quality (all p < 0.05). There was no significant difference in the incidences of adverse events between groups (p = 1.000).
Conclusions: The new-generation IPL was effective and safe in relieving the symptoms and signs of MGD-related dry eye, exhibiting a non-inferior effective rate compared to the traditional IPL. Additionally, Eyesis showed more clinical benefits over E-Eye in alleviating symptoms, increasing tear film stability and improving meibomian gland function.
Keywords: Dry eye; Intense pulsed light; Meibomian gland dysfunction; Tear film.
© 2022. The Author(s).
Figures
Similar articles
-
Intense pulsed light (IPL) therapy for the treatment of meibomian gland dysfunction.Cochrane Database Syst Rev. 2020 Mar 18;3(3):CD013559. doi: 10.1002/14651858.CD013559. Cochrane Database Syst Rev. 2020. PMID: 32182637 Free PMC article.
-
Effect of Intense Pulsed Light Therapy in Dry Eye Disease Caused by Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis.Eye Contact Lens. 2022 Oct 1;48(10):424-429. doi: 10.1097/ICL.0000000000000934. Epub 2022 Sep 5. Eye Contact Lens. 2022. PMID: 36044829
-
Comparative Evaluation in Intense Pulsed Light Therapy Combined with or without Meibomian Gland Expression for the Treatment of Meibomian Gland Dysfunction.Curr Eye Res. 2021 Aug;46(8):1125-1131. doi: 10.1080/02713683.2020.1867750. Epub 2021 Jan 18. Curr Eye Res. 2021. PMID: 33342317 Clinical Trial.
-
Efficacy and safety of intense pulsed light of upper and lower eyelids in Meibomian gland dysfunction: A prospective multicentric study.Eur J Ophthalmol. 2024 May;34(3):700-707. doi: 10.1177/11206721231199121. Epub 2023 Sep 6. Eur J Ophthalmol. 2024. PMID: 37671407
-
Intense pulsed light improves signs and symptoms of dry eye disease due to meibomian gland dysfunction: A randomized controlled study.PLoS One. 2022 Jun 23;17(6):e0270268. doi: 10.1371/journal.pone.0270268. eCollection 2022. PLoS One. 2022. PMID: 35737696 Free PMC article. Clinical Trial.
Cited by
-
Long-Term Impacts of Intense Pulsed Light Therapy on Ocular Surface Health and Tear Film Dynamics in Patients with Dry Eye Disease: Detailed Analysis and Observations Over a 1-Year Follow-Up Period.Ophthalmol Ther. 2024 Oct;13(10):2715-2730. doi: 10.1007/s40123-024-01017-7. Epub 2024 Aug 16. Ophthalmol Ther. 2024. PMID: 39150603 Free PMC article.
-
Current application of intense pulsed light for the management of dry eye disease: A systematic review and meta-analysis.Indian J Ophthalmol. 2024 Feb 1;72(Suppl 2):S183-S190. doi: 10.4103/IJO.IJO_671_23. Epub 2023 Dec 26. Indian J Ophthalmol. 2024. PMID: 38146980 Free PMC article.
-
Impact of dry eye disease treatment on patient quality of life.Front Med (Lausanne). 2024 Feb 28;11:1305579. doi: 10.3389/fmed.2024.1305579. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38482530 Free PMC article. Review.
-
Treatment of Dry Eye Disease (DED) in Asia: Strategies for Short Tear Film Breakup Time-Type DED.Pharmaceutics. 2023 Nov 5;15(11):2591. doi: 10.3390/pharmaceutics15112591. Pharmaceutics. 2023. PMID: 38004570 Free PMC article. Review.
References
-
- Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for. MGD Investig Ophthalmol Vis Sci. 2011;52(4):1994–2005. doi: 10.1167/iovs.10-6997e. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

