HMGB1 in the mPFC governs comorbid anxiety in neuropathic pain
- PMID: 35974316
- PMCID: PMC9382735
- DOI: 10.1186/s10194-022-01475-z
HMGB1 in the mPFC governs comorbid anxiety in neuropathic pain
Abstract
Background: Whether neuroinflammation causes comorbid mood disorders in neuropathic pain remains elusive. Here we investigated the role of high mobility group box 1 protein (HMGB1), a proinflammatory cytokine, in the medial prefrontal cortex (mPFC) in anxiety comorbidity of neuropathic pain.
Methods: Neuropathic pain was induced by partial transection of the infraorbital nerve (p-IONX) or partial sciatic nerve ligation (PSL) in mice and evaluated by measuring nociceptive thresholds to mechanical and heat stimulation. Anxiety-like behaviors were assessed by elevated plus maze, light dark box and open field tests. Aversive or anti-aversive effect was detected by conditioned place preference test. Neuronal activity was evaluated by single-unit and patch clamp recordings. The contribution of mPFC pyramidal neurons to anxiety was further examined by selectively inhibiting them by optogenetics. HMGB1 expression was measured by immunohistochemistry and western blotting. Antagonism of HMGB1 was achieved by injecting anti-HMGB1 monoclonal antibody (mAb) intracerebrally or intraperitoneally.
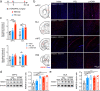
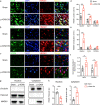
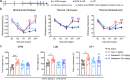
Results: Anxiety-like behaviors were presented earlier after p-IONX than after PSL. HMGB1 expression was upregulated in the mPFC temporally in parallel to anxiety onset, rather than in other regions associated with anxiety. The upregulation of HMGB1 expression and its translocation from the nucleus to cytoplasm in the mPFC occurred predominantly in neurons and were accompanied with activation of microglia and astrocytes. Infusion of anti-HMGB1 mAb into the mPFC during the early and late phases after either p-IONX or PSL alleviated anxiety-like behaviors and aversion without changing pain sensitization, while local infusion of exogenous ds-HMGB1, the proinflammatory form of HMGB1, into the mPFC induced anxiety and aversion but not pain sensitization in naïve mice. In addition to reversing established pain sensitization and anxiety simultaneously, intraperitoneal injection of anti-HMGB1 mAb reduced HMGB1 upregulation and suppressed the hyperexcitability of layer 2/3 pyramidal neurons in the mPFC after p-IONX. Moreover, optogenetic inhibition of mPFC pyramidal neurons alleviated anxiety in p-IONX mice.
Conclusion: These results demonstrate that HMGB1 in the mPFC drives and maintains anxiety comorbidity in neuropathic pain by increasing the excitability of layer 2/3 pyramidal neurons, and justify antagonism of HMGB1, e.g., neutralization by mAb, as a promising therapeutic strategy for neuropathic pain with anxiety comorbidity.
Keywords: Anxiety; Comorbid mood disorders; HMGB1; Neuroinflammation; Neuropathic pain; mPFC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Failde I, Dueñas M, Ribera MV, Gálvez R, Mico JA, Salazar A, de Sola H, Pérez C. Prevalence of central and peripheral neuropathic pain in patients attending pain clinics in Spain: factors related to intensity of pain and quality of life. J Pain Res. 2018;11:1835–1847. doi: 10.2147/JPR.S159729. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

