Modeling the early stages of Alzheimer's disease by administering intracerebroventricular injections of human native Aβ oligomers to rats
- PMID: 35974377
- PMCID: PMC9380371
- DOI: 10.1186/s40478-022-01417-5
Modeling the early stages of Alzheimer's disease by administering intracerebroventricular injections of human native Aβ oligomers to rats
Abstract
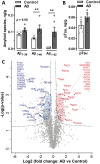
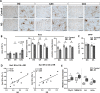
Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disease characterized by the accumulation of aggregated amyloid beta (Aβ) and hyperphosphorylated tau along with a slow decline in cognitive functions. Unlike advanced AD, the initial steps of AD pathophysiology have been poorly investigated, partially due to limited availability of animal models focused on the early, plaque-free stages of the disease. The aim of this study was to evaluate the early behavioral, anatomical and molecular alterations in wild-type rats following intracerebroventricular injections of human Aβ oligomers (AβOs). Bioactive human AD and nondemented control brain tissue extracts were characterized using ELISA and proteomics approaches. Following a bilateral infusion, rats underwent behavioral testing, including the elevated plus maze, social recognition test, Morris water maze and Y-maze within 6 weeks postinjection. An analysis of brain structure was performed with manganese-enhanced MRI. Collected brain tissues were analyzed using stereology, immunohistochemistry, ELISA and qPCR. No sensorimotor deficits affecting motor performance on different maze tasks were observed, nor was spatial memory disturbed in AD rats. In contrast, a significant impairment of social memory became evident at 21 days postinjection. This deficit was associated with a significantly decreased volume of the lateral entorhinal cortex and a tendency toward a decrease in the total brain volume. Significant increase of cleaved caspase-3-positive cells, microglial activation and proinflammatory responses accompanied by altered expression of synaptic markers were observed in the hippocampus of AD rats with immunohistochemical and qPCR approaches at 6 weeks postinjection. Our data suggest that the social memory impairment observed in AβO-injected rats might be determined by neuroinflammatory responses and synaptopathy. An infusion of native oligomeric Aβ in the rat brain represents a feasible tool to model early plaque-free events associated with AD.
Keywords: Alzheimer’s disease; Hippocampus; Lateral entorhinal cortex; Neuroinflammation; Social recognition.
© 2022. The Author(s).
Conflict of interest statement
All authors have no conflicts of interest to declare.
Figures






Similar articles
-
Minocycline effects on memory and learning impairment in the beta-amyloid-induced Alzheimer's disease model in male rats using behavioral, biochemical, and histological methods.Eur J Pharmacol. 2023 Aug 15;953:175784. doi: 10.1016/j.ejphar.2023.175784. Epub 2023 May 11. Eur J Pharmacol. 2023. PMID: 37179042
-
Beta-amyloid pathology in the entorhinal cortex of rats induces memory deficits: implications for Alzheimer's disease.Neuroscience. 2007 Jun 15;147(1):28-36. doi: 10.1016/j.neuroscience.2007.04.011. Epub 2007 May 17. Neuroscience. 2007. PMID: 17499931
-
Prodromal neuroinflammatory, cholinergic and metabolite dysfunction detected by PET and MRS in the TgF344-AD transgenic rat model of AD: a collaborative multi-modal study.Theranostics. 2021 May 3;11(14):6644-6667. doi: 10.7150/thno.56059. eCollection 2021. Theranostics. 2021. PMID: 34093845 Free PMC article.
-
Alzheimer's Disease and Its Possible Evolutionary Origin: Hypothesis.Cells. 2023 Jun 13;12(12):1618. doi: 10.3390/cells12121618. Cells. 2023. PMID: 37371088 Free PMC article. Review.
-
Towards developing a rhesus monkey model of early Alzheimer's disease focusing on women's health.Am J Primatol. 2021 Nov;83(11):e23289. doi: 10.1002/ajp.23289. Epub 2021 May 31. Am J Primatol. 2021. PMID: 34056733 Review.
Cited by
-
In Vivo Seeding of Amyloid-β Protein and Implications in Modeling Alzheimer's Disease Pathology.Biomolecules. 2025 Apr 11;15(4):571. doi: 10.3390/biom15040571. Biomolecules. 2025. PMID: 40305318 Free PMC article. Review.
-
A Peptide Motif Covering Splice Site B in Neuroligin-1 Binds to Aβ and Acts as a Neprilysin Inhibitor.Mol Neurobiol. 2025 Mar;62(3):3244-3257. doi: 10.1007/s12035-024-04475-z. Epub 2024 Sep 11. Mol Neurobiol. 2025. PMID: 39261388 Free PMC article.
-
Regional AT-8 reactive tau species correlate with intracellular Aβ levels in cases of low AD neuropathologic change.Acta Neuropathol. 2024 Feb 14;147(1):40. doi: 10.1007/s00401-024-02691-4. Acta Neuropathol. 2024. PMID: 38353753 Free PMC article.
-
Microglia and Cholesterol Handling: Implications for Alzheimer's Disease.Biomedicines. 2022 Dec 1;10(12):3105. doi: 10.3390/biomedicines10123105. Biomedicines. 2022. PMID: 36551857 Free PMC article. Review.
-
Anti-oxidant activity of coenzyme Q10 against AlCl3/D-galactose in albino rat induced cognitive dysfunctions: Behavioral, biochemical, and BACE-1/GSK-3β alterations.Toxicol Res (Camb). 2024 Aug 19;13(4):tfae131. doi: 10.1093/toxres/tfae131. eCollection 2024 Aug. Toxicol Res (Camb). 2024. PMID: 39165833 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

