MiR-942-5p targeting the IFI27 gene regulates HCT-8 cell apoptosis via a TRAIL-dependent pathway during the early phase of Cryptosporidium parvum infection
- PMID: 35974384
- PMCID: PMC9382849
- DOI: 10.1186/s13071-022-05415-3
MiR-942-5p targeting the IFI27 gene regulates HCT-8 cell apoptosis via a TRAIL-dependent pathway during the early phase of Cryptosporidium parvum infection
Abstract
Background: MicroRNAs (miRNAs) are involved in the regulation of both the innate and adaptive immune response to Cryptosporidium parvum infection. We previously reported that C. parvum upregulated miR‑942‑5p expression in HCT‑8 cells via TLR2/TLR4‑NF‑κB signaling. In the present study, the role of miRNA-942-5p in the regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated HCT-8 cell apoptosis induced by C. parvum was investigated.
Methods: Quantitative real-time polymerase chain reaction, western blotting, flow cytometry, and immunofluorescence were used for analysis.
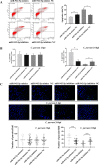
Results: Forced expression of miRNA-942-5p resulted in decreased apoptosis and an increased C. parvum burden in HCT-8 cells. The opposite results were observed using the suppressed expression of miRNA-942-5p. The miRNA-942-5p led to the translational suppression of IFI27 gene through targeting the 3'-untranslated region of the IFI27 gene. Moreover, overexpression of the IFI27 gene produced a high apoptotic ratio and low C. parvum burden. In contrast, a low apoptotic ratio and a high C. parvum burden were observed following downregulation of the IFI27 gene. Both miR-942-5p and the IFI27 gene influenced TRAIL and caspase-8 expression induced by C. parvum in HCT-8 cells. Moreover, TRAIL promoted HCT-8 cell apoptosis in a concentration-dependent manner.
Conclusions: These data suggested that C. parvum induced the downregulation of IFI27 via relief of miR-942-5p-mediated translational suppression. IFI27 downregulation was affected the burden of C. parvum by regulating HCT-8 cell apoptosis through TRAIL-dependent pathways. Future studies should determine the mechanisms by which C. parvum infection increases miR-942-5p expression and the role of miR-942-5p in hosts' anti-C. parvum immunity in vivo.
Keywords: Apoptosis; Cryptosporidium parvum; HCT-8 cell; Parasite burden; miR-942-5p.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51. - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

