Nuclear entry and egress of parvoviruses
- PMID: 35974704
- PMCID: PMC9805091
- DOI: 10.1111/mmi.14974
Nuclear entry and egress of parvoviruses
Abstract
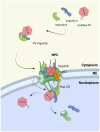
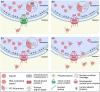
Parvoviruses are small non-enveloped single-stranded DNA viruses, which depend on host cell nuclear transcriptional and replication machinery. After endosomal exposure of nuclear localization sequence and a phospholipase A2 domain on the capsid surface, and escape into the cytosol, parvovirus capsids enter the nucleus. Due to the small capsid diameter of 18-26 nm, intact capsids can potentially pass into the nucleus through nuclear pore complexes (NPCs). This might be facilitated by active nuclear import, but capsids may also follow an alternative entry pathway that includes activation of mitotic factors and local transient disruption of the nuclear envelope. The nuclear entry is followed by currently undefined events of viral genome uncoating. After genome release, viral replication compartments are initiated and infection proceeds. Parvoviral genomes replicate during cellular S phase followed by nuclear capsid assembly during virus-induced S/G2 cell cycle arrest. Nuclear egress of capsids occurs upon nuclear envelope degradation during apoptosis and cell lysis. An alternative pathway for nuclear export has been described using active transport through the NPC mediated by the chromosome region maintenance 1 protein, CRM1, which is enhanced by phosphorylation of the N-terminal domain of VP2. However, other alternative but not yet uncharacterized nuclear export pathways cannot be excluded.
Keywords: import and export; nuclear envelope; nuclear pore complexes; nucleus; parvoviruses.
© 2022 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Figures

References
-
- Abschuetz, A. , Kehl, T. , Geibig, R. , Leuchs, B. , Rommelaere, J. & Régnier‐Vigouroux, A. (2006) Oncolytic murine autonomous parvovirus, a candidate vector for glioma gene therapy, is innocuous to normal and immunocompetent mouse glial cells. Cell and Tissue Research, 325, 423–436. 10.1007/S00441-006-0199-Z/FIGURES/8 - DOI - PubMed
-
- Arora, R. , Malla, W.A. , Tyagi, A. , Mahajan, S. , Sajjanar, B. & Tiwari, A.K. (2021) Canine parvovirus and its non‐structural gene 1 as oncolytic agents: mechanism of action and induction of anti‐tumor immune response. Frontiers in Oncology, 11, 1290. 10.3389/FONC.2021.648873/BIBTEX - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

