Electrophysiological biomarkers of brain function in CDKL5 deficiency disorder
- PMID: 35974796
- PMCID: PMC9374482
- DOI: 10.1093/braincomms/fcac197
Electrophysiological biomarkers of brain function in CDKL5 deficiency disorder
Abstract
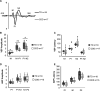
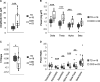
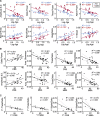
CDKL5 deficiency disorder is a debilitating developmental and epileptic encephalopathy for which no targeted treatment exists. A number of promising therapeutics are under development for CDKL5 deficiency disorder but a lack of validated biomarkers of brain function and clinical severity may limit the ability to objectively assess the efficacy of new treatments as they become available. To address this need, the current study quantified electrophysiological measures in individuals with CDKL5 deficiency disorder and the association between these parameters and clinical severity. Visual and auditory evoked potentials, as well as resting EEG, were acquired across 5 clinical sites from 26 individuals with CDKL5 deficiency disorder. Evoked potential and quantitative EEG features were calculated and compared with typically developing individuals in an age- and sex-matched cohort. Baseline and Year 1 data, when available, were analysed and the repeatability of the results was tested. Two clinician-completed severity scales were used for evaluating the clinical relevance of the electrophysiological parameters. Group-level comparisons revealed reduced visual evoked potential amplitude in CDKL5 deficiency disorder individuals versus typically developing individuals. There were no group differences in the latency of the visual evoked potentials or in the latency or amplitude of the auditory evoked potentials. Within the CDKL5 deficiency disorder group, auditory evoked potential amplitude correlated with disease severity at baseline as well as Year 1. Multiple quantitative EEG features differed between CDKL5 deficiency disorder and typically developing participants, including amplitude standard deviation, 1/f slope and global delta, theta, alpha and beta power. Several quantitative EEG features correlated with clinical severity, including amplitude skewness, theta/delta ratio and alpha/delta ratio. The theta/delta ratio was the overall strongest predictor of severity and also among the most repeatable qEEG measures from baseline to Year 1. Together, the present findings point to the utility of evoked potentials and quantitative EEG parameters as objective measures of brain function and disease severity in future clinical trials for CDKL5 deficiency disorder. The results also underscore the utility of the current methods, which could be similarly applied to the identification and validation of electrophysiological biomarkers of brain function for other developmental encephalopathies.
Keywords: CDKL5 deficiency disorder; biomarkers; developmental encephalopathies; evoked potentials; quantitative EEG.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Muller A, Helbig I, Jansen C, et al. Retrospective evaluation of low long-term efficacy of antiepileptic drugs and ketogenic diet in 39 patients with CDKL5-related epilepsy. Eur J Paediatr Neurol. 2016;20(1):147–151. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
