Kinetics of the photolysis of pyridaben and its main photoproduct in aqueous environments under simulated solar irradiation
- PMID: 35975087
- PMCID: PMC9350664
- DOI: 10.1039/d2ra02601e
Kinetics of the photolysis of pyridaben and its main photoproduct in aqueous environments under simulated solar irradiation
Abstract
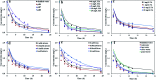
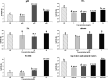
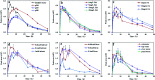
The photolytic fate of pyridaben and its main photolysis product was investigated in different aqueous solutions. Results showed that the photolysis of pyridaben followed pseudo first-order kinetics or the hockey-stick model. In buffer solutions, the half-life of pyridaben was the shortest at pH 4, while the degradation rate within 24 h was the highest at pH 9. Humic acids (HA) at concentrations of 1-20 mg L-1 favored the photolysis of pyridaben while fulvic acids (FA) did not have a significant effect. Nitrate at low concentrations (0.01 mM) accelerated the photolysis and Fe(iii) at high concentrations (0.01 and 0.1 mM) significantly inhibited the photolysis. The photolysis rate of pyridaben in rainwater, tap water, and river water was significantly higher than that in distilled water. The half-lives in distilled water, rainwater, tap water, river water, and pond water were 2.36, 1.36, 1.61, 1.77, and 2.68 h, respectively. Ultra-high-performance liquid chromatography/high-resolution mass spectrometry identified M328 as a photolysis product. The degradation of M328 followed pseudo first-order kinetics in distilled water, buffer solutions and aqueous solutions fortified with HA. The half-lives of M328 were in the range of 7.07-13.95 h. These results are essential for further environmental risk assessment of pyridaben.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

References
-
- Zhu X. Feng X. Yuan C. Cao X. Li J. J. Mol. Catal. A: Chem. 2004;214:293–300. doi: 10.1016/j.molcata.2004.01.001. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

