Highly selective acid-catalyzed olefin isomerization of limonene to terpinolene by kinetic suppression of overreactions in a confined space of porous metal-macrocycle frameworks
- PMID: 35975147
- PMCID: PMC9350587
- DOI: 10.1039/d2sc01561g
Highly selective acid-catalyzed olefin isomerization of limonene to terpinolene by kinetic suppression of overreactions in a confined space of porous metal-macrocycle frameworks
Erratum in
-
Correction: Highly selective acid-catalyzed olefin isomerization of limonene to terpinolene by kinetic suppression of overreactions in a confined space of porous metal-macrocycle frameworks.Chem Sci. 2022 Aug 11;13(33):9787-9790. doi: 10.1039/d2sc90161g. eCollection 2022 Aug 24. Chem Sci. 2022. PMID: 36091891 Free PMC article.
Abstract
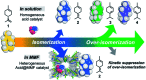
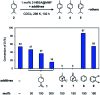
Natural enzymes control the intrinsic reactivity of chemical reactions in the natural environment, giving only the necessary products. In recent years, challenging research on the reactivity control of terpenes with structural diversity using artificial host compounds that mimic such enzymatic reactions has been actively pursued. A typical example is the acid-catalyzed olefin isomerization of (+)-limonene, which generally gives a complex mixture due to over-isomerization to thermodynamically favored isomers. Herein we report a highly controlled conversion of (+)-limonene by kinetic suppression of over-isomerization in a confined space of a porous metal-macrocycle framework (MMF) equipped with a Brønsted acid catalyst. The terminal double bond of (+)-limonene migrated to one neighbor, preferentially producing terpinolene. This reaction selectivity was in stark contrast to the homogeneous acid-catalyzed reaction in bulk solution and to previously reported catalytic reactions. X-ray structural analysis and examination of the reaction with adsorption inhibitors suggest that the reactive substrates may bind non-covalently to specific positions in the confined space of the MMF, thereby inhibiting the over-isomerization reaction. The nanospaces of the MMF with substrate binding ability are expected to enable highly selective synthesis of a variety of terpene compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Correction: Highly selective acid-catalyzed olefin isomerization of limonene to terpinolene by kinetic suppression of overreactions in a confined space of porous metal-macrocycle frameworks.Chem Sci. 2022 Aug 11;13(33):9787-9790. doi: 10.1039/d2sc90161g. eCollection 2022 Aug 24. Chem Sci. 2022. PMID: 36091891 Free PMC article.
-
Novel Porous Crystals with Macrocycle-Based Well-Defined Molecular Recognition Sites.Acc Chem Res. 2020 Mar 17;53(3):632-643. doi: 10.1021/acs.accounts.9b00566. Epub 2020 Jan 23. Acc Chem Res. 2020. PMID: 31970991 Review.
-
Preferential Photoreaction in a Porous Crystal, Metal-Macrocycle Framework: PdII-Mediated Olefin Migration over [2+2] Cycloaddition.J Am Chem Soc. 2018 Dec 5;140(48):16610-16614. doi: 10.1021/jacs.8b08534. Epub 2018 Nov 26. J Am Chem Soc. 2018. PMID: 30407819
-
Cobalt-Catalyzed Regioselective Olefin Isomerization Under Kinetic Control.J Am Chem Soc. 2018 Jun 6;140(22):6873-6882. doi: 10.1021/jacs.8b01815. Epub 2018 May 29. J Am Chem Soc. 2018. PMID: 29781616
-
The Hexameric Resorcinarene Capsule at Work: Supramolecular Catalysis in Confined Spaces.Chemistry. 2019 Apr 1;25(19):4899-4913. doi: 10.1002/chem.201805206. Epub 2019 Jan 23. Chemistry. 2019. PMID: 30499615 Review.
Cited by
-
Micro- and nanochamber array system for single enzyme assays.Sci Rep. 2023 Aug 16;13(1):13322. doi: 10.1038/s41598-023-40544-4. Sci Rep. 2023. PMID: 37587179 Free PMC article.
-
Porous Supramolecular Crystalline Probe that Detects Non-Covalent Interactions Involved in Molecular Recognition of Furanic Compounds.Small. 2024 Dec;20(49):e2405507. doi: 10.1002/smll.202405507. Epub 2024 Jul 30. Small. 2024. PMID: 39076053 Free PMC article.
-
Strain-Inducing Positional Alkene Isomerization.J Am Chem Soc. 2023 Sep 13;145(36):20053-20061. doi: 10.1021/jacs.3c06935. Epub 2023 Aug 30. J Am Chem Soc. 2023. PMID: 37647593 Free PMC article.
-
Electric-Field Catalysis on Carbon Nanotubes in Electromicrofluidic Reactors: Monoterpene Cyclizations.Angew Chem Int Ed Engl. 2025 Jan 21;64(4):e202417333. doi: 10.1002/anie.202417333. Epub 2024 Nov 14. Angew Chem Int Ed Engl. 2025. PMID: 39387156 Free PMC article.
-
Multipoint Coordinative Capture of Olefinic Substrates Utilizing Metal-Carbon Bonds by Macrocyclic Complex.Angew Chem Int Ed Engl. 2025 Aug 25;64(35):e202505734. doi: 10.1002/anie.202505734. Epub 2025 Jul 17. Angew Chem Int Ed Engl. 2025. PMID: 40590189 Free PMC article.
References
-
- Bugg T. D., Introduction to enzyme and coenzyme chemistry, John Wiley & Sons, 2012
LinkOut - more resources
Full Text Sources

