Solvent dielectric delimited nitro-nitrito photorearrangement in a perylenediimide derivative
- PMID: 35975155
- PMCID: PMC9350666
- DOI: 10.1039/d2sc02979k
Solvent dielectric delimited nitro-nitrito photorearrangement in a perylenediimide derivative
Abstract
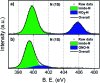
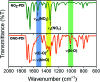
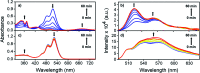
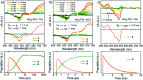
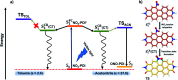
The discovery of vibrant excited-state dynamics and distinctive photochemistry has established nitrated polycyclic aromatic hydrocarbons as an exhilarating class of organic compounds. Herein, we report the atypical photorearrangement of nitro-perylenediimide (NO2-PDI) to nitrito-perylenediimide (ONO-PDI), triggered by visible-light excitation and giving rise to linkage isomers in the polar aprotic solvent acetonitrile. ONO-PDI has been isolated and unambiguously characterized using standard spectroscopic, spectrometric, and elemental composition techniques. Although nitritoaromatic compounds are conventionally considered to be crucial intermediates in the photodissociation of nitroaromatics, experimental evidence for this has not been observed heretofore. Ultrafast transient absorption spectroscopy combined with computational investigations revealed the prominence of a conformationally relaxed singlet excited-state (SCR 1) of NO2-PDI in the photoisomerization pathway. Theoretical transition state (TS) analysis indicated the presence of a six-membered cyclic TS, which is pivotal in connecting the SCR 1 state to the photoproduct state. This article addresses prevailing knowledge gaps in the field of organic linkage isomers and provides a comprehensive understanding of the unprecedented photoisomerization mechanism operating in the case of NO2-PDI.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Light harvesting zinc naphthalocyanine-perylenediimide supramolecular dyads: long-lived charge-separated states in nonpolar media.Phys Chem Chem Phys. 2012 Mar 14;14(10):3612-21. doi: 10.1039/c2cp23285e. Epub 2012 Feb 7. Phys Chem Chem Phys. 2012. PMID: 22311067
-
Interrelations between Linkage Isomers of an Efficient Square-planar Nickel(II) Nitrite Photoswitch in the Solid State.Chemistry. 2023 Dec 22;29(72):e202302629. doi: 10.1002/chem.202302629. Epub 2023 Nov 7. Chemistry. 2023. PMID: 37723126
-
Excited-State Symmetry-Breaking Charge Separation Dynamics in Multibranched Perylene Diimide Molecules.J Phys Chem Lett. 2020 Dec 17;11(24):10329-10339. doi: 10.1021/acs.jpclett.0c03210. Epub 2020 Nov 24. J Phys Chem Lett. 2020. PMID: 33232151
-
Photogeneration of Several Linkage Isomers and Investigation of Forward and Backward Nitro-Nitrito Isomerization Processes in a Palladium Complex.Inorg Chem. 2023 Apr 10;62(14):5531-5542. doi: 10.1021/acs.inorgchem.3c00028. Epub 2023 Mar 29. Inorg Chem. 2023. PMID: 36989116
-
Excited state dynamics and photochemistry of nitroaromatic compounds.Chem Commun (Camb). 2021 Nov 19;57(92):12218-12235. doi: 10.1039/d1cc04999b. Chem Commun (Camb). 2021. PMID: 34735557 Review.
Cited by
-
Unveiling the topology of partially disordered micro-crystalline nitro-perylenediimide with X-aggregate stacking: an integrated approach.Chem Sci. 2023 Nov 23;15(2):490-499. doi: 10.1039/d3sc05514k. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179523 Free PMC article.
-
Single-Component Hydrophilic Terpolymer Thin Film Systems for Imparting Surface Chemical Versatility on Various Substrates.Polymers (Basel). 2023 Dec 21;16(1):44. doi: 10.3390/polym16010044. Polymers (Basel). 2023. PMID: 38201709 Free PMC article.
References
LinkOut - more resources
Full Text Sources

