All-in-one disulfide bridging enables the generation of antibody conjugates with modular cargo loading
- PMID: 35975158
- PMCID: PMC9350601
- DOI: 10.1039/d2sc02198f
All-in-one disulfide bridging enables the generation of antibody conjugates with modular cargo loading
Abstract
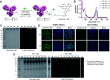
Antibody-drug conjugates (ADCs) are valuable therapeutic entities which leverage the specificity of antibodies to selectively deliver cytotoxins to antigen-expressing targets such as cancer cells. However, current methods for their construction still suffer from a number of shortcomings. For instance, using a single modification technology to modulate the drug-to-antibody ratio (DAR) in integer increments while maintaining homogeneity and stability remains exceptionally challenging. Herein, we report a novel method for the generation of antibody conjugates with modular cargo loading from native antibodies. Our approach relies on a new class of disulfide rebridging linkers, which can react with eight cysteine residues, thereby effecting all-in-one bridging of all four interchain disulfides in an IgG1 antibody with a single linker molecule. Modification of the antibody with the linker in a 1 : 1 ratio enabled the modulation of cargo loading in a quick and selective manner through derivatization of the linker with varying numbers of payload attachment handles to allow for attachment of either 1, 2, 3 or 4 payloads (fluorescent dyes or cytotoxins). Assessment of the biological activity of these conjugates demonstrated their exceptional stability in human plasma and utility for cell-selective cytotoxin delivery or imaging/diagnostic applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
FMD, SJW and DRS are inventors on a patent application relating to the use of TetraDVP linkers for ADC synthesis.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

