Asymmetric synthesis of chromanone lactones via vinylogous conjugate addition of butenolide to 2-ester chromones
- PMID: 35975160
- PMCID: PMC9350614
- DOI: 10.1039/d2sc02541h
Asymmetric synthesis of chromanone lactones via vinylogous conjugate addition of butenolide to 2-ester chromones
Abstract
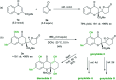
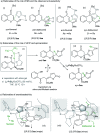
Chiral chromanone lactones are a class of natural products with important biological activity. We report a direct diastereo- and enantioselective vinylogous conjugate addition of butenolide to 2-ester substituted chromones. The transformation proceeded well in the presence of as low as 1 mol% of a chiral N,N'-dioxide/ScIII complex, 3 Å MS and a catalytic amount of hexafluoroisopropanol (HFIP). The scope of Michael acceptors includes a variety of substituted chromones at different positions, and the desired chromanone lactones upon reduction are afforded in good yield and diastereoselectivity, and excellent enantioselectivity (up to 99% ee). The strategy could be used in the concise synthesis of blennolide C and gonytolide A, C and G.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Concise and Stereodivergent Approach to Chromanone Lactones through Copper-Catalyzed Asymmetric Vinylogous Addition of Siloxyfurans to 2-Ester-Substituted Chromones.Angew Chem Int Ed Engl. 2022 Jun 27;61(26):e202203128. doi: 10.1002/anie.202203128. Epub 2022 May 9. Angew Chem Int Ed Engl. 2022. PMID: 35475558
-
Catalytic Asymmetric Vinylogous Conjugate Addition of Butenolide to 2-Ester-Substituted Chromones: Access to Chiral Chromanone Lactones via Trapping of a Copper(I) Enolate by Trimethyl Borate.Org Lett. 2023 Nov 24;25(46):8367-8371. doi: 10.1021/acs.orglett.3c03503. Epub 2023 Nov 14. Org Lett. 2023. PMID: 37962864
-
TMSI-promoted vinylogous Michael addition of siloxyfuran to 2-substituted chromones: a general approach for the total synthesis of chromanone lactone natural products.J Org Chem. 2015 Feb 6;80(3):1632-43. doi: 10.1021/jo502571r. Epub 2015 Jan 12. J Org Chem. 2015. PMID: 25560746
-
Copper-catalyzed enantioselective conjugate addition of organometallic reagents to challenging Michael acceptors.Beilstein J Org Chem. 2020 Feb 17;16:212-232. doi: 10.3762/bjoc.16.24. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32180841 Free PMC article. Review.
-
Diastereo- and enantioselective anti-selective hydrogenation of α-amino-β-keto ester hydrochlorides and related compounds using transition-metal-chiral-bisphosphine catalysts.Chem Rec. 2014 Apr;14(2):235-50. doi: 10.1002/tcr.201300032. Epub 2014 Feb 18. Chem Rec. 2014. PMID: 24550034 Review.
Cited by
-
Regioselective C-H alkylation of anisoles with olefins by cationic imidazolin-2-iminato scandium(iii) alkyl complexes.Chem Sci. 2023 Jan 21;14(12):3132-3139. doi: 10.1039/d2sc06725k. eCollection 2023 Mar 22. Chem Sci. 2023. PMID: 36970095 Free PMC article.
-
Harnessing Dpp-Imine as a Powerful Achiral Cocatalyst to Dramatically Increase the Efficiency and Stereoselectivity in a Magnesium-Mediated Oxa-Michael Reaction.JACS Au. 2023 Dec 21;4(1):164-176. doi: 10.1021/jacsau.3c00584. eCollection 2024 Jan 22. JACS Au. 2023. PMID: 38274262 Free PMC article.
References
-
- McPhee F. Caldera P. S. Bemis G. W. McDonagh A. F. Kuntz I. D. Craik C. S. Biochem. J. 1996;320:681. doi: 10.1042/bj3200681. - DOI - PMC - PubMed
- Bräse S. Encinas A. Keck J. Nising C. F. Chem. Rev. 2009;109:3903. doi: 10.1021/cr050001f. - DOI - PubMed
- Siddiqui I. N. Zahoor A. Hussain H. Ahmed I. Ahmad V. U. Padula D. Draeger S. Schulz B. Meier K. Steinert M. Kurtán T. Flörke U. Pescitelli G. Krohn K. J. Nat. Prod. 2011;74:365. doi: 10.1021/np100730b. - DOI - PubMed
- Kikuchi H. Isobe M. Sekiya M. Abe Y. Hoshikawa T. Ueda K. Kurata S. Katou Y. Oshima Y. Org. Lett. 2011;13:4624. doi: 10.1021/ol2018449. - DOI - PubMed
- El-Elimat T. Figueroa M. Raja A. H. Graf T. N. Swanson S. M. Falkinham III J. O. Wani M. C. Pearce C. J. Oberlies N. H. Eur. J. Org. Chem. 2015:109. doi: 10.1002/ejoc.201402984. - DOI - PMC - PubMed
- Yodsing N. Lekphrom R. Sangsopha W. Aimi T. Boonlue S. Curr. Microbiol. 2018;75:513. doi: 10.1007/s00284-017-1411-y. - DOI - PubMed
- Xie L. Li M. Liu D. Wang X. Wang P. Dai H. Yang W. Liu W. Hu X. Zhao M. Molecules. 2019;24:393. doi: 10.3390/molecules24030393. - DOI - PMC - PubMed
-
- Bröhmer M. C. Bourcet E. Nieger M. Bräse S. Chem.–Eur. J. 2011;17:13706. doi: 10.1002/chem.201102192. - DOI - PubMed
- Masters K. S. Bräse S. Chem. Rev. 2012;112:3717. doi: 10.1021/cr100446h. - DOI - PubMed
- Tietze L. F. Ma L. Reiner J. R. Jackenkroll S. Heidemann S. Chem.–Eur. J. 2013;19:8610. doi: 10.1002/chem.201300479. - DOI - PubMed
- Sudhakar G. Bayya S. Kadam V. D. Nanubolu J. B. Org. Biomol. Chem. 2014;12:5601. doi: 10.1039/C4OB00950A. - DOI - PubMed
- Adachi K. Hasegawa S. Katakawa K. Kumamoto T. Tetrahedron Lett. 2017;58:4479. doi: 10.1016/j.tetlet.2017.10.038. - DOI
-
- Qin T. Porco Jr J. A. Angew. Chem. 2014;126:3171. doi: 10.1002/ange.201311260. - DOI
- Qin T. Skraba-Joiner S. L. Khalil Z. G. Johnson R. P. Capon R. J. Porco Jr J. A. Nat. Chem. 2015;7:234. doi: 10.1038/nchem.2173. - DOI - PMC - PubMed
- Qin T. Iwata T. Ransom T. T. Beutler J. A. Porco Jr J. A. J. Am. Chem. Soc. 2015;137:15225. doi: 10.1021/jacs.5b09825. - DOI - PMC - PubMed
- Wu X. W. Iwata T. Scharf A. Qin T. Reichl K. D. Porco Jr J. A. J. Am. Chem. Soc. 2018;140:5969. doi: 10.1021/jacs.8b02535. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources