A pH-independent electrochemical aptamer-based biosensor supports quantitative, real-time measurement in vivo
- PMID: 35975161
- PMCID: PMC9350589
- DOI: 10.1039/d2sc02021a
A pH-independent electrochemical aptamer-based biosensor supports quantitative, real-time measurement in vivo
Abstract
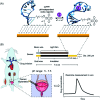
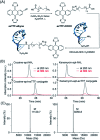
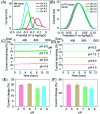
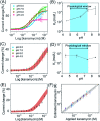
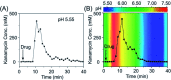
The development of biosensors capable of achieving accurate and precise molecular measurements in the living body in pH-variable biological environments (e.g. subcellular organelles, biological fluids and organs) plays a significant role in personalized medicine. Because they recapitulate the conformation-linked signaling mechanisms, electrochemical aptamer-based (E-AB) sensors are good candidates to fill this role. However, this class of sensors suffers from a lack of a stable and pH-independent redox reporter to support their utility under pH-variable conditions. Here, in response, we demonstrate the efficiency of an electron donor π-extended tetrathiafulvalene (exTTF) as an excellent candidate (due to its good electrochemical stability and no proton participation in its redox reaction) of pH-independent redox reporters. Its use has allowed improvement of E-AB sensing performance in biological fluids under different pH conditions, achieving high-frequency, real-time molecular measurements in biological samples both in vitro and in the bladders of living rats.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Bariya M. Nyein H. Y. Y. Javey A. Nat. Electron. 2018;1:160. doi: 10.1038/s41928-018-0043-y. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

