"It Was Very Comforting to Find Out Right Away." - Patient Perspectives on Point-of-Care Molecular SARS-CoV-2 Testing in Primary Care
- PMID: 35975172
- PMCID: PMC9375998
- DOI: 10.2147/PPA.S372366
"It Was Very Comforting to Find Out Right Away." - Patient Perspectives on Point-of-Care Molecular SARS-CoV-2 Testing in Primary Care
Abstract
Background: The use of point-of-care tests (POCTs) has been a central strategy to cope with the COVID-19 pandemic. Yet, evidence on the application and consequences of POCTs within medical settings is rare.
Purpose: To assess and understand patient perspectives on molecular point-of-care SARS-CoV-2 testing conducted in primary care.
Methods: We conducted a cross-sectional survey study among patients who were tested with a molecular SARS-CoV-2 rapid test (ID NOWTM COVID-19 rapid test, Abbott) in 13 primary care practices in the state of Thuringia (Germany) from February to April 2021. The following aspects were covered in the questionnaire through rating scales and open text formats: test characteristics, trust in test result, consequences of immediate result, cost amount willing to pay and expectations in the future. Open text answers were categorized; quantitative data were analyzed using descriptive statistics and a Mann-Whitney U-test to reveal differences in cost contribution depending on the test result.
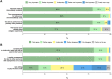
Results: A total of 215 patients from nine family practices and one pediatric practice participated. The immediate availability of the test result was important to the majority of patients (94.3%). 95.7% of patients trusted in their test result. Personal consequences of the immediate test result referred to pandemic measures, certainty of action and reassurance. For further tests, patients were willing to pay between 0€ and 100€ (interquartile range = 10-25€) for the molecular SARS-CoV-2 POCT, regardless of the test result. Expectations of being offered the test again in case of renewed cold symptoms were reported by 96.2%.
Conclusion: Patients highly appreciated molecular SARS-CoV-2 rapid testing conducted in primary care practices. The immediate availability of the test result led to adjustments in patients' behavior and emotional wellbeing. However, potentially challenging for the implementation of POCTs in primary care practices may be the reimbursement of test costs and patients' expectations in future situation.
Keywords: COVID-19; POCT; acceptance; feasibility; rapid test.
© 2022 Matthes et al.
Conflict of interest statement
Ms Anni Matthes reports grants from the Ministry of Education and Research, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures
References
-
- German Federal Ministry of Health. National Testing Strategy SARS-Cov-2 [in German]. Berlin, Germany: German Federal Ministry of Health; 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous

