Surface-tailoring of emulsomes for boosting brain delivery of vinpocetine via intranasal route: in vitro optimization and in vivo pharmacokinetic assessment
- PMID: 35975309
- PMCID: PMC9387308
- DOI: 10.1080/10717544.2022.2110996
Surface-tailoring of emulsomes for boosting brain delivery of vinpocetine via intranasal route: in vitro optimization and in vivo pharmacokinetic assessment
Abstract
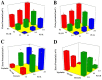
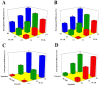
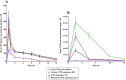
Vinpocetine (VNP), a semisynthetic active pharmaceutical ingredient, is used for oral management of cerebrovascular diseases because of its ability to enhance the blood flow to the brain. However, despite that, the therapeutic application of VNP is restricted due to its reduced bioavailability and diminished brain levels that could be attributed to its low aqueous solubility, short half-life, and presystemic metabolism exposure. Accordingly, the goal of this work was to explore the ability of surface-tailored intranasal emulsomes to boost brain delivery of the drug. A 3221 factorial design was implemented to explore the impact of phospholipid (PL) to solid lipid weight ratio, PL to cholesterol molar ratio, and type of solid lipid on vesicle size, zeta potential, drug entrapment, and release efficiency of the new developed VNP emulsomes. Tailoring of the optimized emulsomal surface formulation was performed using either cationization or PEGylation approaches to boost blood-brain barrier penetration. The pharmacokinetic assessment in rats showed significantly improved bioavailability of VNP emulsomal formulations compared to the oral market product. Additionally, surface-tailored emulsomes exhibited significantly higher brain levels compared to the optimized emulsomes. Based on these findings, the proposed surface-tailored emulsomes could be considered as a promising platform for achieving high brain levels of VNP following intranasal administration.
Keywords: PEGylation; Vinpocetine; brain delivery; cationization; pharmacokinetics; surface-tailored intranasal emulsomes.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




References
-
- Abdel-Mottaleb MM, Lamprecht A. (2011). Standardized in vitro drug release test for colloidal drug carriers using modified USP dissolution apparatus I. Drug Dev Ind Pharm 37:178–84. - PubMed
-
- Ahirrao M, Shrotriya S. (2017). In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. Drug Dev Ind Pharm 43:1686–93. - PubMed
-
- Ahmed TA, Badr-Eldin SM, Ahmed OA, Aldawsari H. (2018). Intranasal optimized solid lipid nanoparticles loaded in situ gel for enhancing trans-mucosal delivery of simvastatin. J Drug Delivery Sci Technol 48:499–508.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
