Injectable Nanoparticle-Based Hydrogels Enable the Safe and Effective Deployment of Immunostimulatory CD40 Agonist Antibodies
- PMID: 35975424
- PMCID: PMC9534946
- DOI: 10.1002/advs.202103677
Injectable Nanoparticle-Based Hydrogels Enable the Safe and Effective Deployment of Immunostimulatory CD40 Agonist Antibodies
Abstract
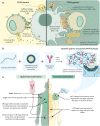
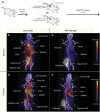
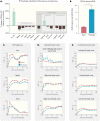
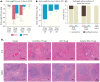
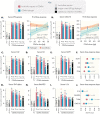
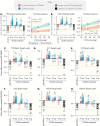
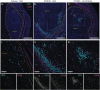
When properly deployed, the immune system can eliminate deadly pathogens, eradicate metastatic cancers, and provide long-lasting protection from diverse diseases. Unfortunately, realizing these remarkable capabilities is inherently risky as disruption to immune homeostasis can elicit dangerous complications or autoimmune disorders. While current research is continuously expanding the arsenal of potent immunotherapeutics, there is a technological gap when it comes to controlling when, where, and how long these drugs act on the body. Here, this study explored the ability of a slow-releasing injectable hydrogel depot to reduce dose-limiting toxicities of immunostimulatory CD40 agonist (CD40a) while maintaining its potent anticancer efficacy. A previously described polymer-nanoparticle (PNP) hydrogel system is leveraged that exhibits shear-thinning and yield-stress properties that are hypothesized to improve locoregional delivery of CD40a immunotherapy. Using positron emission tomography, it is demonstrated that prolonged hydrogel-based delivery redistributes CD40a exposure to the tumor and the tumor draining lymph node (TdLN), thereby reducing weight loss, hepatotoxicity, and cytokine storm associated with standard treatment. Moreover, CD40a-loaded hydrogels mediate improved local cytokine induction in the TdLN and improve treatment efficacy in the B16F10 melanoma model. PNP hydrogels, therefore, represent a facile, drug-agnostic method to ameliorate immune-related adverse effects and explore locoregional delivery of immunostimulatory drugs.
Keywords: biomaterials; cancer; drug delivery; immunotherapy; pharmacokinetics.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Rosenberg S. A., Sci. Transl. Med. 2012, 4, 127ps8; - PMC - PubMed
- b) Alexander W., P T 2016, 41, 185; - PMC - PubMed
- c) Topalian S. L., Hodi F. S., Brahmer J. R., Gettinger S. N., Smith D. C., McDermott D. F., Powderly J. D., Carvajal R. D., Sosman J. A., Atkins M. B., N. Engl. J. Med. 2012, 366, 2443; - PMC - PubMed
- d) Larkin J., Chiarion‐Sileni V., Gonzalez R., Grob J. J., Cowey C. L., Lao C. D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., N. Engl. J. Med. 2015, 373, 23. - PMC - PubMed
-
- a) Binnewies M., Roberts E. W., Kersten K., Chan V., Fearon D. F., Merad M., Coussens L. M., Gabrilovich D. I., Ostrand‐Rosenberg S., Hedrick C. C., Vonderheide R. H., Pittet M. J., Jain R. K., Zou W., Howcroft T. K., Woodhouse E. C., Weinberg R. A., Krummel M. F., Nat. Med. 2018, 24, 541; - PMC - PubMed
- b) Bonaventura P., Shekarian T., Alcazer V., Valladeau‐Guilemond J., Valsesia‐Wittmann S., Amigorena S., Caux C., Depil S., Front. Immunol. 2019, 10, 10. - PMC - PubMed
-
- Zou W., Nat. Rev. Cancer 2005, 5, 263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
