Implantable Electroceutical Approach Improves Myelination by Restoring Membrane Integrity in a Mouse Model of Peripheral Demyelinating Neuropathy
- PMID: 35975427
- PMCID: PMC9661852
- DOI: 10.1002/advs.202201358
Implantable Electroceutical Approach Improves Myelination by Restoring Membrane Integrity in a Mouse Model of Peripheral Demyelinating Neuropathy
Abstract
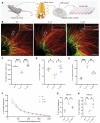
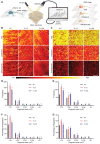
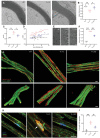
Although many efforts are undertaken to treat peripheral demyelinating neuropathies based on biochemical interventions, unfortunately, there is no approved treatment yet. Furthermore, previous studies have not shown improvement of the myelin membrane at the biomolecular level. Here, an electroceutical treatment is introduced as a biophysical intervention to treat Charcot-Marie-Tooth (CMT) disease-the most prevalent peripheral demyelinating neuropathy worldwide-using a mouse model. The specific electrical stimulation (ES) condition (50 mV mm-1 , 20 Hz, 1 h) for optimal myelination is found via an in vitro ES screening system, and its promyelinating effect is validated with ex vivo dorsal root ganglion model. Biomolecular investigation via time-of-flight secondary ion mass spectrometry shows that ES ameliorates distribution abnormalities of peripheral myelin protein 22 and cholesterol in the myelin membrane, revealing the restoration of myelin membrane integrity. ES intervention in vivo via flexible implantable electrodes shows not only gradual rehabilitation of mouse behavioral phenotypes (balance and endurance), but also restored myelin thickness, compactness, and membrane integrity. This study demonstrates, for the first time, that an electroceutical approach with the optimal ES condition has the potential to treat CMT disease and restore impaired myelin membrane integrity, shifting the paradigm toward practical interventions for peripheral demyelinating neuropathies.
Keywords: Charcot-Marie-Tooth disease; PMP22; cholesterol; electroceuticals; myelin membrane integrity; myelination; peripheral demyelinating neuropathies.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Electroceutical approach ameliorates intracellular PMP22 aggregation and promotes pro-myelinating pathways in a CMT1A in vitro model.Biosens Bioelectron. 2023 Mar 15;224:115055. doi: 10.1016/j.bios.2022.115055. Epub 2022 Dec 30. Biosens Bioelectron. 2023. PMID: 36630746
-
An electroceutical approach enhances myelination via upregulation of lipid biosynthesis in the dorsal root ganglion.Biofabrication. 2022 Jan 6;14(1). doi: 10.1088/1758-5090/ac457c. Biofabrication. 2022. PMID: 34933294
-
Dysregulation of ErbB Receptor Trafficking and Signaling in Demyelinating Charcot-Marie-Tooth Disease.Mol Neurobiol. 2017 Jan;54(1):87-100. doi: 10.1007/s12035-015-9668-2. Epub 2016 Jan 5. Mol Neurobiol. 2017. PMID: 26732592 Free PMC article. Review.
-
Enhanced axonal neuregulin-1 type-III signaling ameliorates neurophysiology and hypomyelination in a Charcot-Marie-Tooth type 1B mouse model.Hum Mol Genet. 2019 Mar 15;28(6):992-1006. doi: 10.1093/hmg/ddy411. Hum Mol Genet. 2019. PMID: 30481294 Free PMC article.
-
Charcot-Marie-Tooth disease and related inherited neuropathies.Medicine (Baltimore). 1996 Sep;75(5):233-50. doi: 10.1097/00005792-199609000-00001. Medicine (Baltimore). 1996. PMID: 8862346 Review.
Cited by
-
Highly Bioadaptable Hybrid Conduits with Spatially Bidirectional Structure for Precision Nerve Fiber Regeneration via Gene Therapy.Adv Sci (Weinh). 2024 May;11(19):e2309306. doi: 10.1002/advs.202309306. Epub 2024 Mar 14. Adv Sci (Weinh). 2024. PMID: 38483934 Free PMC article.
-
Advances in Mass Spectrometry-Based Single Cell Analysis.Biology (Basel). 2023 Mar 2;12(3):395. doi: 10.3390/biology12030395. Biology (Basel). 2023. PMID: 36979087 Free PMC article. Review.
-
Silver electroceutical technology to treat sarcopenia.Proc Natl Acad Sci U S A. 2023 Aug 15;120(33):e2300036120. doi: 10.1073/pnas.2300036120. Epub 2023 Aug 7. Proc Natl Acad Sci U S A. 2023. PMID: 37549292 Free PMC article.
References
-
- Barreto L. C., Oliveira F. S., Nunes P. S., de Franca Costa I. M., Garcez C. A., Goes G. M., Neves E. L., de Souza Siqueira Quintans J., de Souza Araujo A. A., Neuroepidemiology 2016, 46, 157. - PubMed
-
- a) Brennan K. M., Bai Y., Shy M. E., Neurosci. Lett. 2015, 596, 14; - PubMed
- b) Murphy S. M., Laura M., Fawcett K., Pandraud A., Liu Y. T., Davidson G. L., Rossor A. M., Polke J. M., Castleman V., Manji H., Lunn M. P., Bull K., Ramdharry G., Davis M., Blake J. C., Houlden H., Reilly M. M., J. Neurol., Neurosurg. Psychiatry 2012, 83, 706. - PMC - PubMed
-
- Morena J., Gupta A., Hoyle J. C., Int. J. Mol. Sci. 2019, 20, 3419. - PubMed
-
- a) Passage E., Norreel J. C., Noack‐Fraissignes P., Sanguedolce V., Pizant J., Thirion X., Robaglia‐Schlupp A., Pellissier J. F., Fontes M., Nat. Med. 2004, 10, 396; - PubMed
- b) Pareyson D., Reilly M. M., Schenone A., Fabrizi G. M., Cavallaro T., Santoro L., Vita G., Quattrone A., Padua L., Gemignani F., Visioli F., Laurà M., Radice D., Calabrese D., Hughes R. A. C., Solari A., Lancet Neurol. 2011, 10, 320. - PubMed
-
- a) Fledrich R., Abdelaal T., Rasch L., Bansal V., Schutza V., Brugger B., Luchtenborg C., Prukop T., Stenzel J., Rahman R. U., Hermes D., Ewers D., Mobius W., Ruhwedel T., Katona I., Weis J., Klein D., Martini R., Bruck W., Muller W. C., Bonn S., Bechmann I., Nave K. A., Stassart R. M., Sereda M. W., Nat. Commun. 2018, 9, 3025; - PMC - PubMed
- b) Zhou Y., Bazick H., Miles J. R., Fethiere A. I., Salihi M. O. A., Fazio S., Tavori H., Notterpek L., Exp. Neurol. 2019, 321, 113031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
