Adiponectin gene therapy prevents islet loss after transplantation
- PMID: 35975481
- PMCID: PMC9465193
- DOI: 10.1111/jcmm.17515
Adiponectin gene therapy prevents islet loss after transplantation
Abstract
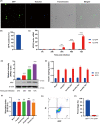
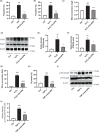
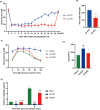
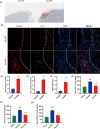
Significant pancreatic islet dysfunction and loss shortly after transplantation to the liver limit the widespread implementation of this procedure in the clinic. Nonimmune factors such as reactive oxygen species and inflammation have been considered as the primary driving force for graft failure. The adipokine adiponectin plays potent roles against inflammation and oxidative stress. Previous studies have demonstrated that systemic administration of adiponectin significantly prevented islet loss and enhanced islet function at post-transplantation period. In vitro studies indicate that adiponectin protects islets from hypoxia/reoxygenation injury, oxidative stress as well as TNF-α-induced injury. By applying adenovirus mediated transfection, we now engineered islet cells to express exogenous adiponectin gene prior to islet transplantation. Adenovirus-mediated adiponectin transfer to a syngeneic suboptimal islet graft transplanted under kidney capsule markedly prevented inflammation, preserved islet graft mass and improved islet transplant outcomes. These results suggest that adenovirus-mediated adiponectin gene therapy would be a beneficial clinical engineering approach for islet preservation in islet transplantation.
Keywords: Adiponectin; gene therapy; hypoxia/reoxygenation injury; inflammation; islet transplantation; oxidative stress.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
P‐OB is founder and CEO of the biotech company Biocrine AB.
Figures
References
-
- Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner‐Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59(6):817‐820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 21RCYJ0046/Program for Overseas High-Level Talents Introduction of Sichuan Province of China
- 139180012/Center of Excellence-International Collaboration Initiative Grant of West China Hospital
- ZYGD18017/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18014/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- 2018M643487/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Medical

