Important roles of transporters in the pharmacokinetics of anti-viral nucleoside/nucleotide analogs
- PMID: 35975669
- PMCID: PMC9506706
- DOI: 10.1080/17425255.2022.2112175
Important roles of transporters in the pharmacokinetics of anti-viral nucleoside/nucleotide analogs
Abstract
Introduction: Nucleoside analogs are an important class of antiviral agents. Due to the high hydrophilicity and limited membrane permeability of antiviral nucleoside/nucleotide analogs (AVNAs), transporters play critical roles in AVNA pharmacokinetics. Understanding the properties of these transporters is important to accelerate translational research for AVNAs.
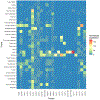
Areas covered: The roles of key transporters in the pharmacokinetics of 25 approved AVNAs were reviewed. Clinically relevant information that can be explained by the modulation of transporter functions is also highlighted.
Expert opinion: Although the roles of transporters in the intestinal absorption and renal excretion of AVNAs have been well identified, more research is warranted to understand their roles in the distribution of AVNAs, especially to immune privileged compartments where treatment of viral infection is challenging. P-gp, MRP4, BCRP, and nucleoside transporters have shown extensive impacts in the disposition of AVNAs. It is highly recommended that the role of transporters should be investigated during the development of novel AVNAs. Clinically, co-administered inhibitors and genetic polymorphism of transporters are the two most frequently reported factors altering AVNA pharmacokinetics. Physiopathology conditions also regulate transporter activities, while their effects on pharmacokinetics need further exploration. Pharmacokinetic models could be useful for elucidating these complicated factors in clinical settings.
Keywords: Antiviral agents; drug transporter; drug–drug interaction; genetic polymorphism; nucleoside/nucleotide analogs; pharmacokinetics.
Figures

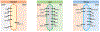
References
-
- Vardanyan RS, Hruby VJ. Chapter 34 - Antiviral Drugs. In: Vardanyan R, Hruby V, editors. Synthesis of Best-Seller Drugs. Boston: Academic Press; 2016. p. 687–736.
-
- Jordheim LP, Durantel D, Zoulim F, et al. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov. 2013. Jun;12(6):447–64. - PubMed
-
* A review highlights the different approaches used in the development of these drugs.
-
- Parkinson FE, Damaraju VL, Graham K, et al. Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr Top Med Chem. 2011;11(8):948–72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
