Signaling by reactive molecules and antioxidants in legume nodules
- PMID: 35975700
- PMCID: PMC9826421
- DOI: 10.1111/nph.18434
Signaling by reactive molecules and antioxidants in legume nodules
Abstract
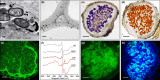
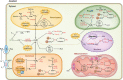
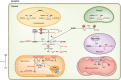
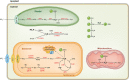
Legume nodules are symbiotic structures formed as a result of the interaction with rhizobia. Nodules fix atmospheric nitrogen into ammonia that is assimilated by the plant and this process requires strict metabolic regulation and signaling. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are involved as signal molecules at all stages of symbiosis, from rhizobial infection to nodule senescence. Also, reactive sulfur species (RSS) are emerging as important signals for an efficient symbiosis. Homeostasis of reactive molecules is mainly accomplished by antioxidant enzymes and metabolites and is essential to allow redox signaling while preventing oxidative damage. Here, we examine the metabolic pathways of reactive molecules and antioxidants with an emphasis on their functions in signaling and protection of symbiosis. In addition to providing an update of recent findings while paying tribute to original studies, we identify several key questions. These include the need of new methodologies to detect and quantify ROS, RNS, and RSS, avoiding potential artifacts due to their short lifetimes and tissue manipulation; the regulation of redox-active proteins by post-translational modification; the production and exchange of reactive molecules in plastids, peroxisomes, nuclei, and bacteroids; and the unknown but expected crosstalk between ROS, RNS, and RSS in nodules.
Keywords: antioxidants; legume-rhizobium symbiosis; nitrogen fixation; post-translational modifications; reactive nitrogen species; reactive oxygen species; reactive sulfur species; redox signaling.
© 2022 The Authors. New Phytologist © 2022 New Phytologist Foundation.
Figures

References
-
- Alesandrini F, Mathis R, Van de Sype G, Hérouart D, Puppo A. 2003. Possible roles for a cysteine protease and hydrogen peroxide in soybean nodule development and senescence. New Phytologist 158: 131–138.
-
- Andrio E, Marino D, Marmeys A, Dunoyer de Segonzac M, Damiani I, Genre A, Huguet S, Frendo P, Puppo A, Pauly N. 2013. Hydrogen peroxide‐regulated genes in the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytologist 198: 190–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

