The proportion of randomized controlled trials that inform clinical practice
- PMID: 35975784
- PMCID: PMC9427100
- DOI: 10.7554/eLife.79491
The proportion of randomized controlled trials that inform clinical practice
Abstract
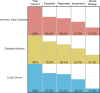
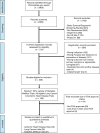
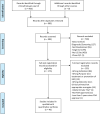
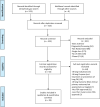
Prior studies suggest that clinical trials are often hampered by problems in design, conduct, and reporting that limit their uptake in clinical practice. We have described 'informativeness' as the ability of a trial to guide clinical, policy, or research decisions. Little is known about the proportion of initiated trials that inform clinical practice. We created a cohort of randomized interventional clinical trials in three disease areas (ischemic heart disease, diabetes mellitus, and lung cancer) that were initiated between January 1, 2009 and December 31, 2010 using ClinicalTrials.gov. We restricted inclusion to trials aimed at answering a clinical question related to the treatment or prevention of disease. Our primary outcome was the proportion of clinical trials fulfilling four conditions of informativeness: importance of the clinical question, trial design, feasibility, and reporting of results. Our study included 125 clinical trials. The proportion meeting four conditions for informativeness was 26.4% (95% CI 18.9-35.0). Sixty-seven percent of participants were enrolled in informative trials. The proportion of informative trials did not differ significantly between our three disease areas. Our results suggest that the majority of randomized interventional trials designed to guide clinical practice possess features that may compromise their ability to do so. This highlights opportunities to improve the scientific vetting of clinical research.
Keywords: clinical trials; informative research; medicine; none; research ethics.
© 2022, Hutchinson et al.
Conflict of interest statement
NH, HM No competing interests declared, DZ received payment as consultant for National Library of Medicine, NIH, for scientific advice to ClinicalTrials.gov and received grants from the Greenwall Foundation, JK received consulting fees from Amylyx Inc and payments from Biomarin. JK participated on Data Safety Monitoring Boards for NIAID and Ultragenyx
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

