A survey study of the use of a subcutaneous implantable cardioverter-defibrillator in various clinical scenarios by expert electrophysiologists in Poland
- PMID: 35975797
- PMCID: PMC10129257
- DOI: 10.5603/CJ.a2022.0073
A survey study of the use of a subcutaneous implantable cardioverter-defibrillator in various clinical scenarios by expert electrophysiologists in Poland
Abstract
Background: A subcutaneous implantable cardioverter-defibrillator (S-ICD) has become a recognized alternative to a traditional transvenous implantable cardioverter-defibrillator (T-ICD). Despite the growing evidence of non-inferiority of S-ICD, there are no clear clinical guidelines for selection of either of the two available systems. The aim of the study was to analyze the decisions made in predefined typical clinical scenarios by Polish cardiologists experienced in the use of both S-ICDs and T-ICDs.
Methods: A group of 30 experts of cardiac electrotherapy experienced in the use of S-ICDs was recruited and invited to participate in a web-based anonymous survey. The survey questions regarded the proposed therapy in various but typical clinical scenarios.
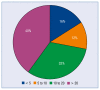
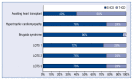
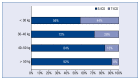
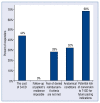
Results: From the invited 30 experts representing 18 clinical centers, 25 completed the survey. 72% of them declared that the number of S-ICDs implanted at their center during the preceding 12 months exceeded 10, and 40% - that it was over 20. Rates of responders preferring S-ICD or T-ICD in various clinical scenarios are reported and discussed in detail.
Conclusions: Significant divergence of opinion exists among Polish experts regarding the use of a subcutaneous cardioverter-defibrillator. It is especially pronounced on the issue of the use of the system in middle-age patients, in case of complications of the hitherto ICD therapy, or the need of upgrading the existing cardiac implantable electronic device.
Keywords: implantable cardioverter-defibrillator; subcutaneous implantable cardioverter-defibrillator; sudden cardiac death; ventricular fibrillation; ventricular tachycardia.
Conflict of interest statement
Figures

References
-
- Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36(41):2793–2867. doi: 10.1093/eurheartj/ehv316. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

