Resolving the structure of V3O7·H2O and Mo-substituted V3O7·H2O
- PMID: 35975830
- PMCID: PMC9370211
- DOI: 10.1107/S2052520622006473
Resolving the structure of V3O7·H2O and Mo-substituted V3O7·H2O
Abstract
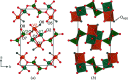
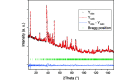
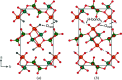
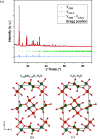
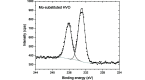
Vanadate compounds, such as V3O7·H2O, are of high interest due to their versatile applications as electrode material for metal-ion batteries. In particular, V3O7·H2O can insert different ions such as Li+, Na+, K+, Mg2+ and Zn2+. In that case, well resolved crystal structure data, such as crystal unit-cell parameters and atom positions, are needed in order to determine the structural information of the inserted ions in the V3O7·H2O structure. In this work, fundamental crystallographic parameters, i.e. atomic displacement parameters, are determined for the atoms in the V3O7·H2O structure. Furthermore, vanadium ions were substituted by molybdenum in the V3O7·H2O structure [(V2.85Mo0.15)O7·H2O] and the crystallographic positions of the molybdenum ions and their oxidation state are elucidated.
Keywords: electrode material; hydrated vanadate; powder X-ray diffraction; powder neutron diffraction; vanadium oxide.
open access.
Figures
References
-
- Gao, S., Chen, Z., Wei, M., Wei, K. & Zhou, H. (2009). Electrochim. Acta, 54, 1115–1118.
-
- He, P., Quan, Y., Xu, X., Yan, M., Yang, W., An, Q., He, L. & Mai, L. (2017). Small, 13, 1702551. - PubMed
-
- Hoelzel, M., Senyshyn, A., Juenke, N., Boysen, H., Schmahl, W. & Fuess, H. (2012). Nucl. Instrum. Methods Phys. Res. A, 667, 32–37.
-
- Huang, X., Rui, X., Hng, H. H. & Yan, Q. (2015). Part. Part. Syst. Charact. 32, 276–294.
-
- Kuhn, A., Pérez-Flores, J. C., Prado-Gonjal, J., Morán, E., Hoelzel, M., Díez-Gómez, V., Sobrados, I., Sanz, J. & García-Alvarado, F. (2022). Chem. Mater. 34, 694–705.
LinkOut - more resources
Full Text Sources

