Synthesis and Stability Studies of a Simplified, Thiazole-Containing Macrocycle of the Anticancer Agent Salarin C
- PMID: 35975906
- PMCID: PMC9746118
- DOI: 10.1021/acs.orglett.2c02326
Synthesis and Stability Studies of a Simplified, Thiazole-Containing Macrocycle of the Anticancer Agent Salarin C
Abstract
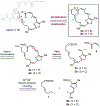
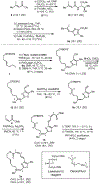
While salarin C (SalaC) is a potent marine cytotoxin, Kashman demonstrated that congeners which had undergone Wasserman rearrangement exhibit little to no cytotoxicity. Given that thiazoles are known to undergo Wasserman rearrangement at a significantly reduced rate, we hypothesized that a thiazole-containing SalaC would exhibit greater stability without significantly altering the macrocyclic conformation. Herein, we describe the synthesis of a simplified, thiazole-containing macrocycle which demonstrates significantly improved stability under identical aerobic conditions.
Figures

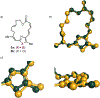


Similar articles
-
Enantioselective Total Synthesis of the Marine Macrolides Salarins A and C.J Am Chem Soc. 2024 Mar 27;146(12):8456-8463. doi: 10.1021/jacs.3c14553. Epub 2024 Mar 13. J Am Chem Soc. 2024. PMID: 38479352
-
Synthesis and cytotoxicity evaluation of thiazole derivatives obtained from 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene- 3-carbonitrile.Acta Pharm. 2017 Dec 20;67(4):495-510. doi: 10.1515/acph-2017-0040. Acta Pharm. 2017. PMID: 29337677
-
Synthesis and Photooxidation of the Trisubstituted Oxazole Fragment of the Marine Natural Product Salarin C.Org Lett. 2017 May 5;19(9):2306-2309. doi: 10.1021/acs.orglett.7b00845. Epub 2017 Apr 20. Org Lett. 2017. PMID: 28425712
-
Synthetic and Medicinal Perspective of Fused-Thiazoles as Anticancer Agents.Anticancer Agents Med Chem. 2021;21(11):1379-1402. doi: 10.2174/1871520620666200728133017. Anticancer Agents Med Chem. 2021. PMID: 32723259 Review.
-
Thiazole-containing compounds as therapeutic targets for cancer therapy.Eur J Med Chem. 2020 Feb 15;188:112016. doi: 10.1016/j.ejmech.2019.112016. Epub 2019 Dec 28. Eur J Med Chem. 2020. PMID: 31926469 Review.
References
-
- Bishara A; Rudi A; Aknin M; Neumann D; Ben-Califa N; Kashman Y Salarins A and B and Tulearin A: New Cytotoxic Sponge-Derived Macrolides. Org. Lett 2008, 10, 153–156. - PubMed
-
- Bishara A; Rudi A; Aknin M; Neumann D; Ben-Califa N; Kashman Y Salarin C, a new cytotoxic sponge-derived nitrogenous macrolide. Tetrahedron Lett. 2008, 49, 4355–4358.
-
- Bishara A; Rudi A; Aknin M; Neumann D; Ben-Califa N; Kashman Y Taumycins A and B, Two Bioactive Lipodepsipeptides from the Madagascar Sponge Fascaplysinopsis sp. Org. Lett 2008, 10, 4307–4309. - PubMed
-
- Bishara A; Rudi A; Aknin M; Neumann D; Ben-Califa N; Kashman Y Salarins D–J, seven new nitrogenous macrolides from the madagascar sponge Fascaplysinopsis sp. Tetrahedron 2010, 66, 4339–4345.
-
- Bishara A; Rudi A; Goldberg I; Aknin M; Kashman Y Tulearins A, B, and C; structures and absolute configurations. Tetrahedron Lett. 2009, 50, 3820–3822.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

