TGFβ1 regulates HRas-mediated activation of IRE1α through the PERK-RPAP2 axis in keratinocytes
- PMID: 35975910
- PMCID: PMC9486931
- DOI: 10.1002/mc.23453
TGFβ1 regulates HRas-mediated activation of IRE1α through the PERK-RPAP2 axis in keratinocytes
Abstract
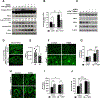
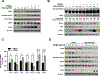
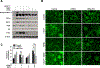
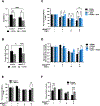
Transforming Growth Factor β1 (TGFβ1) is a critical regulator of tumor progression in response to HRas. Recently, TGFβ1 has been shown to trigger ER stress in many disease models; however, its role in oncogene-induced ER stress is unclear. Oncogenic HRas induces the unfolded protein response (UPR) predominantly via the Inositol-requiring enzyme 1α (IRE1α) pathway to initiate the adaptative responses to ER stress, with importance for both proliferation and senescence. Here, we show a role of the UPR sensor proteins IRE1α and (PKR)-like endoplasmic reticulum kinase (PERK) to mediate the tumor-suppressive roles of TGFβ1 in mouse keratinocytes expressing mutant forms of HRas. TGFβ1 suppressed IRE1α phosphorylation and activation by HRas both in in vitro and in vivo models while simultaneously activating the PERK pathway. However, the increase in ER stress indicated an uncoupling of ER stress and IRE1α activation by TGFβ1. Pharmacological and genetic approaches demonstrated that TGFβ1-dependent dephosphorylation of IRE1α was mediated by PERK through RNA Polymerase II Associated Protein 2 (RPAP2), a PERK-dependent IRE1α phosphatase. In addition, TGFβ1-mediated growth arrest in oncogenic HRas keratinocytes was partially dependent on PERK-induced IRE1α dephosphorylation and inactivation. Together, these results demonstrate a critical cross-talk between UPR proteins that is important for TGFβ1-mediated tumor suppressive responses.
Keywords: ER stress; HRas; IRE1α; PERK; TGFβ1; proliferation; unfolded protein response.
© 2022 Wiley Periodicals LLC.
Figures


Similar articles
-
ER stress and distinct outputs of the IRE1α RNase control proliferation and senescence in response to oncogenic Ras.Proc Natl Acad Sci U S A. 2017 Sep 12;114(37):9900-9905. doi: 10.1073/pnas.1701757114. Epub 2017 Aug 28. Proc Natl Acad Sci U S A. 2017. PMID: 28847931 Free PMC article.
-
Targeting IRE1α and PERK in the endoplasmic reticulum stress pathway attenuates fatty acid-induced insulin resistance in bovine hepatocytes.J Dairy Sci. 2022 Aug;105(8):6895-6908. doi: 10.3168/jds.2021-21754. Epub 2022 Jul 13. J Dairy Sci. 2022. PMID: 35840398
-
PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK- and IRE1α-mediated unfolded protein response.Nat Cell Biol. 2012 Nov;14(11):1223-30. doi: 10.1038/ncb2593. Epub 2012 Oct 28. Nat Cell Biol. 2012. PMID: 23103912 Free PMC article.
-
Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy.Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1417-1427. doi: 10.1093/abbs/gmab131. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34664059 Review.
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
Cited by
-
The FBXW7-RPAP2 Axis Controls the Growth of Hepatocellular Carcinoma Cells and Determines the Fate of Liver Cell Differentiation.Adv Sci (Weinh). 2025 Apr;12(13):e2404718. doi: 10.1002/advs.202404718. Epub 2025 Feb 11. Adv Sci (Weinh). 2025. PMID: 39932049 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

