Reduced activity of vertically acting motoneurons during convergence
- PMID: 35975913
- PMCID: PMC9485007
- DOI: 10.1152/jn.00111.2022
Reduced activity of vertically acting motoneurons during convergence
Abstract
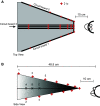
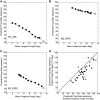
Previous studies have revealed unexpected relationships between the firing rates of horizontally acting motoneurons and vergence. During a vergence task, for example, antidromically identified abducens internuclear neurons show a negative correlation between vergence angle and firing rate, which is the opposite of the modulation displayed by the medial rectus motoneurons to which they project. For a given horizontal eye position, medial rectus motoneurons discharge at a higher rate if the eyes are converged than if the same eye position is reached during a task that requires version; paradoxically, however, the horizontal rectus eye muscles show corelaxation during vergence. These complex and unexpected relationships inspired the present author to investigate whether the tonic firing rates of vertically acting motoneurons in oculomotor nucleus are correlated with vergence angle. Monkeys were trained to fixate a single, randomly selected, visual target among an array of 60 red plus-shaped LEDs, arranged at 12 different distances in three-dimensional space. The targets were arranged to permit dissociation of vertical eye position and vergence angle. Here I report, for the first time, that most vertically acting motoneurons in oculomotor nucleus show a significant negative correlation between tonic firing rate and vergence angle. This suggests the possibility that there may be a general corelaxation of extraocular muscles during vergence.NEW & NOTEWORTHY An array of 60 plus-shaped LEDs, positioned at various locations in three-dimensional space, was used to elicit conjugate and disjunctive saccades while single neurons in oculomotor nucleus were recorded from rhesus monkeys. This study demonstrates that most vertically acting motoneurons in oculomotor nucleus discharge at a lower rate when the eyes are converged.
Keywords: monkey; motoneurons; neurophysiology; oculomotor nucleus; vergence.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author.
Figures





Similar articles
-
Characteristics of antidromically identified oculomotor internuclear neurons during vergence and versional eye movements.J Neurophysiol. 1994 Mar;71(3):1111-27. doi: 10.1152/jn.1994.71.3.1111. J Neurophysiol. 1994. PMID: 8201406
-
Dynamic properties of medial rectus motoneurons during vergence eye movements.J Neurophysiol. 1992 Jan;67(1):64-74. doi: 10.1152/jn.1992.67.1.64. J Neurophysiol. 1992. PMID: 1552323
-
Abducens internuclear neurons carry an inappropriate signal for ocular convergence.J Neurophysiol. 1989 Jul;62(1):70-81. doi: 10.1152/jn.1989.62.1.70. J Neurophysiol. 1989. PMID: 2754482
-
[Central oculomotor circuits].Rev Neurol (Paris). 1985;141(5):349-70. Rev Neurol (Paris). 1985. PMID: 3901182 Review. French.
-
The primate oculomotor system. I. Motoneurons. A synthesis of anatomical, physiological, and clinical data.Hum Neurobiol. 1982;1(2):77-85. Hum Neurobiol. 1982. PMID: 6764460 Review.
Cited by
-
Orienting Gaze Toward a Visual Target: Neurophysiological Synthesis with Epistemological Considerations.Vision (Basel). 2025 Jan 14;9(1):6. doi: 10.3390/vision9010006. Vision (Basel). 2025. PMID: 39846622 Free PMC article. Review.
-
Midbrain lesion-induced disconjugate gaze: a unifying circuit mechanism of ocular alignment?J Neurol. 2024 May;271(5):2844-2849. doi: 10.1007/s00415-023-12155-6. Epub 2024 Feb 14. J Neurol. 2024. PMID: 38353747 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

